Percent Composition & Empirical Formulas Chemistry Presentation
advertisement

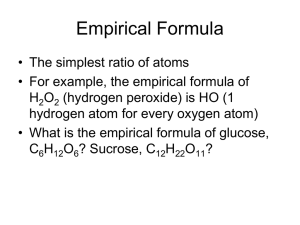
7.3 Percent composition and chemical formulas Percent composition • The relative amount of mass of each element in a compound, expressed in % Calculating percent composition % composition of an element = mass of element/mass of compound X 100 • The sum of all the % compositions of all the elements must equal 100% Percent composition • We don’t know the mass of the element , but we can find the percent composition using the formula and the molar mass of a substance percent composition = grams of element in 1 mole molar mass of compound 100 8.20 g Mg combines completely with 5.40 g O. What is the percent composition of this compound? 8.20 g Mg combines completely with 5.40 g O. What is the percent composition of this compound? • Add the masses of the elements • 8.20 g + 5.40 g = 13.60 g • Divide the mass of each element by the total mass and multiply by 100 (make it a percent) • 8.20 g Mg / 13.60 g = 60.3 % Mg • That makes the % composition of O 39.7 % 222.6 g N combines completely with 77.4 g O. What is the percent composition of each element? 222.6 g N combines completely with 77.4 g O. What is the percent composition of each element? • Total weight of compound is 300 g • 222.6 g/300 g X 100 = 74.2 % N • 77.4 g/300 g X 100 = 25.8% O • 74.2 %+ 25.8 % = 100% Calculate the percent composition of HCN. Calculate the percent composition of HCN. • • • • • • • Molar mass H = 1 g Molar mass C = 12 g Molar mass N = 14 g Total molar mass = 27 g 1 g H/27 g X 100 = 3.7% H 12 g C/27 g X 100 = 44.4% C 14 g N/27 g X 100 = 51.9% N Using the % composition of H in HCN (3.7%), calculate the amount of H in 378 g HCN. Using the % composition of H in HCN (3.7%), calculate the amount of H in 378 g HCN. • 378 g HCN X 3.7 g H/100 g HCN 378 g HCN 3.7 g H 100 g HCN 14 g H Conversion factor… There are 3.7 g of H in each 100 g HCN Empirical formulas • Percent composition can be used to calculate the empirical formula for a compound – Empirical formulas are the lowest whole number ratios of the atoms in a compound – Empirical formulas may or may not be the actual formula when we are dealing with molecules • For example, hydrogen peroxide, H2O2 has an empirical formula of HO, but doesn’t occur in nature that way Other cases where empirical formulas don’t tell us the composition of a molecule • Empirical formula CH – Molecular formula C2H2 (ethyne or acetylene) – Molecular formula C6H6 (benzene) • Empirical formula CH2O – Molecular formula C2H4O2 (ethanoic acid) – Molecular formula C6H12O6 (glucose) What is the empirical formula of a compound which is 25.9% N and 74.1% O? 25.9 g N 1 mol N 14 g N 1.85 mol N 74.1 g O 1 mol O 16 g O 4.63 mol O The ratio of moles of N to moles of O is 1.85/4.63, or 1/2.5. In whole numbers, this is a ratio of 2/5. Therefore, the empirical formula for this compound is N2O5. Calculate the empirical formula of a compound which is 43.64% P and 56.36% O. Calculate the empirical formula of a compound which is 43.64% P and 56.36% O. 43.64 g P 1 mol P 30.97 g P 1.409 mol P 56.36 g O 1 mol O 16 g O 3.523 mol O The ratio of moles of P to moles of O is 1.409/3.523, or 1/2.5. In whole numbers, this is a ratio of 2/5. Therefore, the empirical formula for this compound is P2O5. A compound is 2.477 g Mn and 1.323 g O. What is the empirical formula? A compound is 2.477 g Mn and 1.323 g O. What is the empirical formula? 2.477 g Mn 1 mol Mn 59.94 g Mn 1.323 g O 1 mol O 16 g O .0413 mol Mn .0827 mol O The ratio of moles of Mn to moles of O is .0413/.0827, or 1/2. Therefore, the empirical formula for this compound is MnO2. Methyl butanoate has a percent composition of: 58.8 % C; 9.8 % H; and 31.4 % O. Its molar mass is 102 grams. Want is its molecular formula? Methyl butanoate has a percent composition of: 58.8 % C; 9.8 % H; and 31.4 % O. Its molar mass is 102 grams. Want is its molecular formula? • .588 X 102 g = 59.98 g C • .098 X 102 g = 9.99 g H • .314 X 102 g = 32.03 g O These numbers look awfully suspicious! The first is 5 moles C, the second is 10 moles H, and the third is 2 moles O. So the molecular formula is C5H10O2