BB_presentation
advertisement

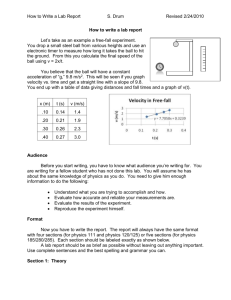
How to find the Density of some Ball Bearings By David Hewitt Apparatus Needed • • • • Displacement can Measuring cylinder Mass balance At least four ball bearings of the same size (more is better) Outline of the Experiment • To find density: Density = Mass Volume • Mass will be measured using a mass balance. • Volume will be found using a displacement cylinder. • Uncertainties will be then be calculated to find an uncertainty for the final value. Step 1: Finding the Mass • Weighing 10 ball bearings and dividing by 10 gives a lower uncertainty than just weighing one, as shown: – Mass of 10 ball bearings: 164.1g – Resolution to 0.1g – Thus % uncertainty = 0.1/164.1*100 = 0.060938% – Mass of 1 ball bearing = 164.1/10 = 16.41g (16.41g ± 0.060938%) Step 2: Finding the Volume • Finding the volume of 3 ball bearings (more would displace too much water) and dividing by 3 works exactly like when finding mass: – Volume of 3 ball bearings: 6.2cm3 – Resolution to 0.1cm3 – Thus % uncertainty = 0.1/6.2*100 = 1.612903% – Volume of 1 ball bearing = 6.2/3 = 2.067cm3 (2.067cm3 ± 1.621903%) Step 3: Some Final Calculations • Density = 16.41/2.067 = 7.940323g/cm3 • % uncertainty = %u of mass + %u of volume (dividing values means ADD % uncertainties). = 0.060938 + 1.612903 = 1.673841% • Uncertainty = value*(%uncertainty/100) = 7.940323 * 0.01673841 = 0.1329 • Quote final value with uncertainty to 1 s.f.: (7.9 ± 0.1) g/cm3