Orbital Diagrams
advertisement

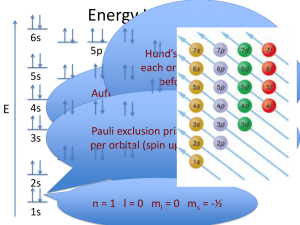
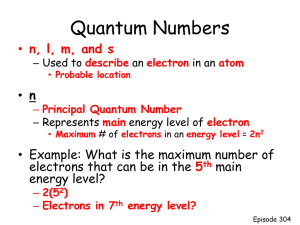
Orbital Diagrams • Orbitals are the places where electrons are placed. • Orbitals reside outside of the nucleus • Orbitals possess energy • The orbital angular momentum is balanced with the electrical forces of attraction between the electrons and protons • This balance keeps the electron in the orbit Orbital Diagrams are based on the SchrÖdinger Equation Solutions for the SchrÖdinger Equation have 3 variables • First variable is called n, principle quantum number and has positive integer values of 1, 2, 3, …. • Second variable is called l, and has positive values of 0, 1, 2,…(n-1); this variable describes the shape of the orbital • Third variable is called m, and has both positive and negative values of – l 0 + l Orbital Diagrams • Main Energy Levels – Rows in the periodic table (quantum designation, n) • Each row contains orbital types (shapes, quantum designation, l) • Orbital types come in 4 styles • • • • s – “basketball shape” (l =0) p – “dumbell shape (l =1) d – complex shape (l =2) f – even more complex shape (l =3) Orbital Diagrams • Ground Rules – Each row represents a different energy level (like the floors in an apartment building) – Within each row there are a different number of kinds of orbitals – Orbitals also have different energy levels with the lowest being the s and the highest being the f Orbital Diagrams • The number of different kinds of orbitals in an energy level is given by the row number. • • • • • Row 1 has 1 kind of orbital, s Row 2 has 2 kinds of orbitals, s and p Row 3 has 3 kinds of orbitals, s, p, and d Row 4 has 4 kinds of orbitals, s, p, d, and f How many kinds of orbital and what would they be called for Row 5? Orbital Diagrams • How many kinds of orbital and what would they be called for Row 5? • There would be 5 kinds of orbitals, s, p, d, f, and g • Do any known elements have electron(s) in the g suborbit? None that are presently known. Orbital Diagrams • How are electrons placed in the energy levels of an atom? • Electrons are always placed in the lowest possible energy level available. This means that the first electron is always placed in the first energy level (n=1) • Electrons are always placed in the lowest energy suborbital s<p<d<f Orbital Diagrams • There is a “trick” in determining the lowest energy state using the numerical value for n and l. • Add (n+ l) • Lowest sum is the lowest energy level and the place where electrons are placed next • If it is a “tie”, then the lowest value of n is the lower energy state Orbital Diagrams • How many orbitals of each kind can be present in an energy level? – s, one (1) – p, three (3) – d, five (5) – f, seven (7) Orbital Diagrams • How many electrons can be placed in any single orbital? TWO (2) This means that the total number of electrons that can be placed in each suborbital is: • • • • s, 2 electrons p, 6 electrons d, 10 electrons f, 14 electrons Orbital Diagrams • Do I really have to know all of these things so that I can write electron configurations? • No; the periodic table is written in such a way that all of these rules can be simplified. Also, there is an easy pneumonic to assist in writing electron configurations. Summary of Orbital Diagrams Energy Level Suborbitals 1 s 2 s p s p d 3 # of Orbital number electrons l= l=0 2 l=0 l=1 l=0 l=1 l=2 2 6 2 6 10 Total # of electrons 2 2n 2 8 18 Summary of Orbital Diagrams Energy Level Suborbitals # of Orbital number electrons l= Total # of electron s 2 2n 4 s p d f 0 1 2 3 2 6 10 14 32 5 s p d F g 0 1 2 3 4 2 6 10 14 18 50 1s 2s 2p These are 3s 3p 3d not used 4s 4p 4d 4f 5s 5p 5d 5f 5g 6s 6p 6d 6f 6g 6h 7s 7p 7d 7f 7g 7h 7i Orbital Diagram for A Nitrogen Atom N 1s 2s 2p 3s Orbital Diagram for A Nitrogen Atom N 1s 2s 2p 3s Why do the arrows point in opposite directions? They represent paired electrons with opposite “spin”. Orbital Diagram for A Nitrogen Atom N 1s 2s 2p 3s Why are the 3/2p electrons written in separate orbitals? This is an example of Hund’s rule of maximum multiplicity. Orbital Diagram for A Nitrogen Atom N 1s 2s 2p 3s Which means? Electrons are negatively charged. They don’t like to get together unless it is absolutely necessary. Orbital Diagram for A Nitrogen Atom N 1s 2s 2p 3s The 2p is the lowest available energy state so all three electrons must be placed here. But there are 3 places open and each one is equivalent (scientific word is “degenerate”) Orbital Diagram for A Nitrogen Atom N 1s 2s 2p 3s So, an electron is placed in the lowest available space until each space has one electron. Only then will electrons begin to pair. Orbital Diagram for A Fluorine Atom F 1s 2s 2p 3s Orbital Diagram for A Magnesium Atom Mg 1s 2s 2p 3s Writing Orbital Diagrams Write the orbital diagram for the electrons in an oxygen atom. Solution Write the orbital diagram for the electrons in an oxygen atom. 1s 2s 2p 3s Writing Orbital Diagrams Write the orbital diagram for the electrons in an iron atom. Solution Write the orbital diagram for the electrons in an iron atom. 1s 2s 2p 3d 3s 3p 4s Solution • Note that the 4s level is filled before the 3d. Why is that? Remember the n + l rule? – For the 4s level, n+ l = 4 + 0 = 4 – For the 3d level, n+ l = 3 + 2 = 5 – That means the 4s is lower in energy than the 3d, so the 4s is filled first. – What happens if there is a “tie”? Then the lower value of n “wins”.