The Evolving Landscape for Treatment
of Diabetic Macular Edema
Rajiv Shah, MD
Wills Eye Institute, Philadelphia, Pennsylvania
A REPORT FROM THE 2012 ANNUAL MEETING OF THE
ASSOCIATION FOR RESEARCH IN VISION AND OPHTHALMOLOGY
© 2012 Direct One Communications, Inc. All rights reserved.
1
Diabetic Macular Edema (DME)
Clinically significant DME is a major cause of vision
loss in patients with diabetic retinopathy.1,2
As the prevalence of diabetes grows worldwide, the
potential loss of vision from DME poses significant
quality-of-life and socioeconomic concerns.3,4
The treatment of DME using focal/grid macular laser
therapy was established by the Early Treatment
Diabetic Retinopathy Study (ETDRS).5
This standard eventually was challenged in a study
by the Diabetic Retinopathy Clinical Research
Network (DRCR.net).6
© 2012 Direct One Communications, Inc. All rights reserved.
2
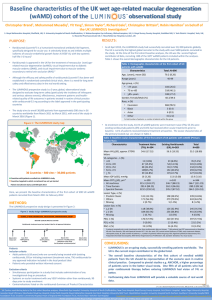
The RESTORE Trial7
RESTORE was the first large, randomized clinical
trial to compare macular laser treatment alone,
ranibizumab alone, and ranibizumab in combination
with laser therapy.
The RESTORE results demonstrated that
ranibizumab used alone or with focal/grid macular
laser therapy provided superior visual acuity
outcomes over focal/grid macular laser treatment
alone in patients with DME.
At 1 year, no differences were detected between the
results achieved with ranibizumab alone and those
from combination ranibizumab/laser therapy.
© 2012 Direct One Communications, Inc. All rights reserved.
3
Continuing Research
The seeds of the DRCR.net trial in 20106 and the
RESTORE trial in 20117 have grown into many
diverse studies and randomized clinical trials that
are trying to determine the best practices for
managing DME.
A growing collection of research showed that the
standard of care as established by the ETDRS over
20 years ago will continue to evolve in the years
ahead.
© 2012 Direct One Communications, Inc. All rights reserved.
4
Factors Predicting Successful
Bevacizumab Therapy in DME
© 2012 Direct One Communications, Inc. All rights reserved.
5
Levels of Blood Markers
Rusu et al8 evaluated the effect of hemoglobin A1c
(HbA1c) level on bevacizumab response during DME
treatment.
Their retrospective chart review evaluated the effect
of HbA1c level on the visual characteristics of 28
patients with DME who were given two consecutive
intravitreal injections of bevacizumab 53 days apart.
© 2012 Direct One Communications, Inc. All rights reserved.
6
Levels of Blood Markers: Results
Using regression modeling, they found that central
foveal thickness, as assessed by optical coherence
tomography (OCT), improved among patients in the
bevacizumab arm as compared with controls not
receiving bevacizumab.
Patients with worse diabetic control (as assessed by
higher HbA1c levels) were less likely to respond to
bevacizumab therapy.
Thus, clinicians must consider the HbA1c level when
they contemplate using bevacizumab to treat
patients with DME.
© 2012 Direct One Communications, Inc. All rights reserved.
7
Prediction of Fellow-Eye Response
Karth et al9 performed a retrospective chart review of
28 DME patients to predict patient response to
intravitreal bevacizumab.
By assessing OCT central thickness of one eye of a
DME patient treated with bevacizumab, they tried to
predict the success of treating the fellow eye.
» Using regression analysis, they found that 21% of the
reduction in central thickness in the fellow eye might be
explained by the response of the first treated eye.
Further validation in larger prospective trials is
needed before treatment efficacy can be gauged by
the response to initial treatment of one eye.
© 2012 Direct One Communications, Inc. All rights reserved.
8
Ranibizumab Monotherapy in DME:
The RISE and RIDE Trials
© 2012 Direct One Communications, Inc. All rights reserved.
9
Ranibizumab Monotherapy in DME:
Background
VEGF plays a role in the creation of retinal ischemia
and increased vascular permeability, which results in
macular edema.10,11
Smaller trials have demonstrated the superiority of
ranibizumab monotherapy over laser therapy alone
in treating DME.7
The RISE and RIDE trials were double-masked,
sham-controlled, multicenter, phase III trials that
evaluated the impact of monthly ranibizumab
injections on DME.12,13
© 2012 Direct One Communications, Inc. All rights reserved.
10
Ranibizumab Monotherapy in DME:
Methods
The RISE and RIDE trials had a parallel design that
compared the use of 0.3 or 0.5 mg of ranibizumab
given monthly for 24 months with sham therapy in
patients who underwent macular laser therapy.
After 3 months of monthly injections of
ranibizumab, grid macular rescue laser therapy was
initiated if either central foveal thickness exceeded
250 µm or worsened by 50 µm or more from the
previous month.
© 2012 Direct One Communications, Inc. All rights reserved.
11
Ranibizumab Monotherapy in DME:
RISE Results
For the RISE trial, 377 patients were randomized to
receive sham therapy (n = 127) or 0.3 mg (n = 125)
or 0.5 mg (n = 125) of ranibizumab.
Baseline patient characteristics were similar across
the three arms.
At 24 months, gains of > 15 ETDRS letters occurred
in:
» 18.1% of patients given sham injections
» 44.8% of those injected with 0.3 mg of ranibizumab
» 39.2% of those given 0.5 mg of ranibizumab
© 2012 Direct One Communications, Inc. All rights reserved.
12
Ranibizumab Monotherapy in DME:
RIDE Results
In the RIDE cohort, 382 patients were randomized
to receive sham therapy (n = 130) or 0.3 mg
(n = 125) or 0.5 mg (n = 127) of ranibizumab.
Baseline patient characteristics were similar across
the three arms.
At 24 months, gains of > 15 ETDRS letters were
noted in:
» 12.3% of sham-injected patients
» 33.6% of those given 0.3 mg of ranibizumab
» 45.7% of patients given 0.5 mg of ranibizumab
© 2012 Direct One Communications, Inc. All rights reserved.
13
Ranibizumab Monotherapy in DME:
RISE and RIDE Results
Significant improvements in macular edema were
noted on OCT in both ranibizumab arms of both
trials.
Diabetic retinopathy was less likely to worsen in
ranibizumab-treated patients.
In the RISE cohort, panretinal photocoagulation
eventually was needed to control progression to
proliferative diabetic retinopathy in:
» 11% of the sham-injected group
» 0% of the group given 0.3 mg of ranibizumab
» 0.8% of those given 0.5 mg of ranibizumab
© 2012 Direct One Communications, Inc. All rights reserved.
14
Ranibizumab Monotherapy in DME:
RISE and RIDE Results
In the RIDE cohort, panretinal photocoagulation
eventually was needed by:
» 12.3% of the sham-injected group
» 1.6% of the group given 0.3 mg of ranibizumab
» 1.6% of those given 0.5 mg of ranibizumab
In both trials, patients treated with ranibizumab had
significantly fewer macular laser procedures.
Over 24 months, a mean of 1.8 laser procedures were
needed in the sham-injected treatment groups,
compared with a mean of 1.6 laser procedures in the
ranibizumab therapy cohorts.
© 2012 Direct One Communications, Inc. All rights reserved.
15
Ranibizumab Monotherapy in DME:
RISE and RIDE Safety Findings
The risk of endophthalmitis from repeated
intraocular injections is a concern, especially in a
diabetic population.
In both the RISE and RIDE trials, encompassing a
total of 10,584 injections, endophthalmitis occurred
in four ranibizumab-treated patients—a reassuringly
low rate.
The total mortality from vascular or unknown
causes, nonfatal myocardial infarctions, and nonfatal
cerebrovascular accidents was 4.9%–5.5% in shaminjected patients and 2.4%–8.8% in ranibizumabtreated patients.
© 2012 Direct One Communications, Inc. All rights reserved.
16
Ranibizumab Monotherapy in DME:
Clinical Impact
The results of both the RISE and RIDE trials helped
to establish monthly ranibizumab monotherapy as
an efficacious and sustainable treatment for DME,
which was associated with low rates of ocular and
systemic complications.
In August 2012, the FDA approved the use of
ranibizumab monotherapy for the treatment of
DME.
© 2012 Direct One Communications, Inc. All rights reserved.
17
Ranibizumab Monotherapy in DME:
Vision Changes in Diabetic Patients
The relationship between DME and diabetes was
established in epidemiologic studies.1,2
The impact of disease beyond visual acuity still must
be assessed.
Turpcu et al14 presented data at the 2012 ARVO
meeting to help validate one such metric, the
National Eye Institute Visual Function
Questionnaire-25 (NEI VFQ-25), as it relates to
vision gains in diabetic patients in the RISE and
RIDE trials.
© 2012 Direct One Communications, Inc. All rights reserved.
18
Ranibizumab Monotherapy in DME:
Vision Changes in Diabetic Patients
The NEI VFQ-25 evaluates patient-reported visual
function in daily activities.
It was given to patients in the RISE and RIDE
cohorts at baseline and at 6, 12, 18, and 24 months.
Results from each trial were evaluated separately;
investigators reported on the 12-month results.
The subgroup of 15 letters gained was evaluated via
regression modeling.
It corresponded with an NEI VFQ-25 composite
score of 8.7 points (95% confidence interval [CI],
5.9–11.5) for the RIDE cohort and 8.3 points (95%
CI, 5.8–10.7) for the RISE cohort.
© 2012 Direct One Communications, Inc. All rights reserved.
19
Ranibizumab Monotherapy in DME:
Vision Changes in Diabetic Patients
The endpoint of 15 letters gained remains a
meaningful vision outcome that has become a
standard in randomized clinical trials of retinal
disease since publication of the
» The Anti-VEGF Antibody for the Treatment of
Predominantly Classic Choroidal Neovascularization (CNV)
in Age-Related Macular Degeneration (AMD; ANCHOR)
trial15
» The Minimally Classic/Occult Trial of the Anti-VEGF
Antibody Ranibizumab in the Treatment of Neovascular
AMD (MARINA)16
The NEI VFQ-25 provides a consistent and validated
patient-reported metric to evaluate outcomes.
© 2012 Direct One Communications, Inc. All rights reserved.
20
Ranibizumab Monotherapy in DME:
Impact on Legal Blindness
Varma and others17 evaluated the theoretical impact
of monthly ranibizumab injections on reducing legal
blindness and visual impairment among nonHispanic white and Hispanic individuals with centerinvolving DME in the United States.
The model evaluated the effect of ranibizumab
treatment on the development of legal blindness
from DME over 2 years.
© 2012 Direct One Communications, Inc. All rights reserved.
21
Ranibizumab Monotherapy in DME:
Impact on Legal Blindness
Legal blindness was defined as a visual acuity letter
score ≤ 38 in the better-seeing eye (approximate
Snellen equivalent, 20/200 or worse).
Visual impairment was defined as a visual acuity
letter score ≤ 68 in the better-seeing eye
(approximate Snellen equivalent, worse than 20/40).
Incident cases of DME were estimated based on the
US population in 2011, the prevalence of diagnosed
diabetes, and the 1-year incidence of clinically
significant DME with visual impairment
The impact of ranibizumab therapy was based on the
results of the RISE and RIDE clinical trials.
© 2012 Direct One Communications, Inc. All rights reserved.
22
Ranibizumab Monotherapy in DME:
Impact on Legal Blindness
The model predicted 17,007 incident cases of DME
with visual impairment in 2011.
» Without ranibizumab therapy, 281 (95% CI, 72–655) cases
of legal blindness were expected over 2 years.
» Monthly ranibizumab treatment reduced this number by
81% (95% CI, 77%–84%) to 54 (95% CI, 14–127) cases.
» In all, 227 (95% CI, 59–531) cases were avoided.
According to this simulation, monthly intravitreal
administration of ranibizumab substantially reduced
legal blindness and visual impairment within 2 years
after DME diagnosis in non-Hispanic white and
Hispanic patients.
© 2012 Direct One Communications, Inc. All rights reserved.
23
Higher-Dose Ranibizumab Therapy
in Patients with DME
© 2012 Direct One Communications, Inc. All rights reserved.
24
Higher-Dose Ranibizumab Therapy in DME:
0.5 mg vs 1 mg
Ferrone and Jonisch18 compared 0.5 mg of
ranibizumab with 1 mg of ranibizumab for the
treatment of DME.
After receiving a loading dose of three consecutive
monthly injections, patients were reevaluated every
other month according to an as-needed treatment
protocol during the first year.
During the second year, patients were seen every
month and received as-needed monthly injections of
ranibizumab.
In all, 42 eyes (0.5 mg, 20 eyes; 1 mg, 22 eyes) with
similar baseline characteristics were evaluated.
© 2012 Direct One Communications, Inc. All rights reserved.
25
Higher-Dose Ranibizumab Therapy in DME:
0.5 mg vs 1 mg
The group given 1 mg of ranibizumab experienced an
average gain of 10.4 ETDRS letters over 2 years.
The 0.5-mg group gained an average of 7.0 ETDRS
letters over the same period.
There was no significant difference in the number of
letters gained between the two groups.
© 2012 Direct One Communications, Inc. All rights reserved.
26
Higher-Dose Ranibizumab Therapy in DME:
0.5 mg vs 1 mg
The mean central foveal thickness was 192 µm in the
1-mg group and 216 µm in the 0.5-mg group at the
end of 2 years, a nonsignificant difference.
An average of 12.0 injections was given to patients in
the 1-mg group and 12.9 injections were given to
those in the 0.5-mg group over 2 years, a
nonsignificant difference.
© 2012 Direct One Communications, Inc. All rights reserved.
27
Higher-Dose Ranibizumab Therapy in DME:
0.5 mg vs 1 mg
These results suggested that patients with DME
benefit from treatment with either 0.5 mg or 1 mg of
ranibizumab.
Among patients given the two doses, no significant
difference was seen in the:
» Number of ETDRS letters gained
» Percentage of patients gaining > 15 letters
» Central foveal thickness (as assessed by OCT)
» Average number of ranibizumab injections needed
© 2012 Direct One Communications, Inc. All rights reserved.
28
Higher-Dose Ranibizumab Therapy in DME:
READ 3 Trial: 0.5 mg vs 2 mg
The READ 3 trial evaluated the use of an even higher
dose of ranibizumab versus the standard 0.5-mg
dose given to manage DME.19,20
In all, 152 eyes with DME were randomized to
receive 0.5 mg or 2 mg of ranibizumab in three
consecutive monthly doses.
Patients found to have a central foveal thickness
> 250 µm were retreated.
© 2012 Direct One Communications, Inc. All rights reserved.
29
Higher-Dose Ranibizumab Therapy in DME:
READ 3 Trial: 0.5 mg vs 2 mg
Data from the READ 3 trial showed a mean change
in best corrected visual acuity (BCVA) from baseline
to month 6—the primary endpoint—of:
» +7.46 ETDRS letters in the 2-mg group
» +8.69 ETDRS letters in the 0.5-mg group.19
The mean reduction in OCT-determined central
foveal thickness was:
» –163.86 μm in the 2-mg group
» –169.27 μm in the 0.5-mg group
No serious ocular or systemic adverse events were
reported in either treatment group.
© 2012 Direct One Communications, Inc. All rights reserved.
30
Higher-Dose Ranibizumab Therapy in DME:
READ 3 Trial: 0.5 mg vs 2 mg
These results show that monthly intravitreal
injection of 0.5 mg or 2 mg of ranibizumab is
similarly effective in managing DME.
The 6-month duration of this study is probably
insufficient to detect differences in efficacy between
the two doses.
Longer, larger, randomized prospective trials are
needed to determine any potential differences
between them.
© 2012 Direct One Communications, Inc. All rights reserved.
31
Higher-Dose Ranibizumab Therapy in DME:
READ 3 Trial: 0.5 mg vs 2 mg
The READ 3 investigators also presented data for the
fellow-eye subgroup.20
If the fellow eye contained clinically significant DME,
it was randomized to receive the opposite dose of
ranibizumab.
A total of 44 patients were eligible for this study.
No notable differences on an inter- or intrapatient
basis were noted between the 0.5 mg and 2 mg of
ranibizumab with respect to BCVA or OCTdetermined central foveal thickness.
© 2012 Direct One Communications, Inc. All rights reserved.
32
Higher-Dose Ranibizumab Therapy in DME:
READ 3 Trial: 0.5 mg vs 2 mg
Another READ 3 evaluation entailed the detection
and retreatment of DME based on time-domain (TD)
versus spectral-domain (SD) OCT.21
» The study investigated treatment decisions made according
to results obtained with different OCT modalities after the
first 6 months of the trial.
Retreatment with ranibizumab was performed if:
» A central foveal thickness > 250 µm was noted using Stratus
TD-OCT (Carl Zeiss Meditec; Dublin, CA).
and/or
» Intraretinal or subretinal fluid was detected using Spectralis
SD-OCT (Heidelberg Engineering GmbH; Heidelberg,
Germany).
© 2012 Direct One Communications, Inc. All rights reserved.
33
Higher-Dose Ranibizumab Therapy in DME:
READ 3 Trial: 0.5 mg vs 2 mg
DME could be present in eyes even when central
foveal thickness was within the acceptable range on
TD-OCT.
SD-OCT could detect intraretinal and subretinal
fluid missed by the TD-OCT system.
Thus, SD-OCT may be a more sensitive imaging
modality for guiding treatment decisions in DME.
Visual acuity outcomes were not evaluated.
Future trials should evaluate whether detection of
DME using SD-OCT or TD-OCT ultimately leads to
better visual outcomes.
© 2012 Direct One Communications, Inc. All rights reserved.
34
Safety and Effectiveness of Combined
Ranibizumab/Laser Therapy in DME
© 2012 Direct One Communications, Inc. All rights reserved.
35
Combined Ranibizumab/Laser Therapy:
RESTORE Extension Study
Two-year safety and efficacy data from the
RESTORE Extension Study22 involved 240 patients
from the RESTORE cohort treated with ranibizumab
on an as-needed basis with or without laser therapy
according to ETDRS guidelines.
Patients were retreated if they experienced a
decrease in BCVA because of DME progression that
was confirmed by clinical evaluation, OCT, or other
anatomic and clinical assessments.7
Patients were treated at monthly intervals until they
again reached stable visual acuity.
© 2012 Direct One Communications, Inc. All rights reserved.
36
Combined Ranibizumab/Laser Therapy:
RESTORE Extension Study
The gains in BCVA that were observed during the
first 12 months were maintained at month 24.
No safety signals were noted in either arm.
The average number of injections in the ranibizumab
monotherapy arm was 3.9, compared with 3.5 in the
combined ranibizumab/laser therapy arm.
As with the RISE and RIDE data, results from the
extension study of RESTORE further emphasized
both safety and continued efficacy of ranibizumab in
treating DME at 24 months.
© 2012 Direct One Communications, Inc. All rights reserved.
37
Combined Ranibizumab/Laser Therapy:
REVEAL Study
The 12-month results of the REVEAL study23 further
corroborated the results of the RESTORE trial.
The REVEAL study followed an Asian cohort with
DME to evaluate the safety and efficacy of:
» 0.5 mg of ranibizumab alone in 133 patients
» 0.5 mg of ranibizumab in combination with laser therapy in
132 patients
» Laser treatment alone in 131 patients.
After receiving three monthly injections, the patients
were treated as needed if they experienced declines
in BCVA and/or DME progression, as found on OCT.
© 2012 Direct One Communications, Inc. All rights reserved.
38
Combined Ranibizumab/Laser Therapy:
REVEAL Study
As in the RESTORE trial, ranibizumab used alone
and with laser therapy resulted in similar results
(gains of 5.9 and 5.7 ETDRS letters, respectively).
Both treatments were superior to laser therapy alone
(gain of 1.4 ETDRS letters).
The mean number of injections needed was 7.8 in
the ranibizumab monotherapy group and 7.4 in
patients given combination therapy.
Ranibizumab given alone or with laser therapy
offered the best outcomes with respect to bestcorrected central foveal thickness.
© 2012 Direct One Communications, Inc. All rights reserved.
39
Combined Ranibizumab/Laser Therapy:
Clinical Implicatons
Together, these results helped to reaffirm that
ranibizumab monotherapy or in combination with
focal/grid macular laser therapy is superior to laser
therapy alone in treating DME patients.
Further, patients receiving ranibizumab experienced
a durable 12- to 24-month clinical benefit of therapy.
As in the RISE and RIDE trials, no significant ocular
or systemic safety signals were detected.
Adding laser therapy to ranibizumab did not lower
the injection burden over time when compared with
the use of ranibizumab alone.
© 2012 Direct One Communications, Inc. All rights reserved.
40
Dexamethasone Intravitreal Implant
in Patients with DME
© 2012 Direct One Communications, Inc. All rights reserved.
41
Dexamethasone Intravitreal Implant
Zucchiatti et al24 assessed the efficacy and safety of
dexamethasone intravitreal implants in patients with
refractory DME.
The retrospective trial evaluated a single intravitreal
injection of the implant in nine eyes with DME that
had previously been treated with intraocular antiVEGF therapy or with corticosteroid and/or laser
therapy.
Investigators used SD-OCT to further evaluate the
retina.
© 2012 Direct One Communications, Inc. All rights reserved.
42
Dexamethasone Intravitreal Implant
Administration of the dexamethasone intravitreal
implant improved the central foveal thickness until
month 4 post injection.
Improvements in BCVA were maintained until
month 4 post injection.
Cataract progression did not occur.
One patient experienced an elevation in intraocular
pressure, which was treated successfully with topical
therapy.
The investigators considered the results promising.
© 2012 Direct One Communications, Inc. All rights reserved.
43
Aflibercept in Patients with DME
© 2012 Direct One Communications, Inc. All rights reserved.
44
Aflibercept in DME:
Background
Aflibercept is an FDA-approved anti-VEGF therapy
for exudative age-related macular edema.
Its role in treating patients with DME has not yet
been fully established.
This recombinant fusion protein, comprising the key
VEGF-binding domains of human VEGF receptors 1
and 2, possesses a:
» Higher binding affinity for VEGF receptors than does
ranibizumab or bevacizumab
» Binding capacity for placental growth factors 1 and 2, which
contribute to excessive vascular permeability and retinal
neovascularization.25,26
© 2012 Direct One Communications, Inc. All rights reserved.
45
Aflibercept in DME:
DA VINCI Trial
In the DA VINCI trial,27 a total of 221 patients with
center-involving DME were randomized to receive:
» 0.5 mg of aflibercept every 4 weeks
» 2 mg of aflibercept every 4 weeks
» 2 mg of aflibercept every 4 weeks for 3 months, followed by
2 mg given every 8 weeks
» 2 mg of aflibercept every 4 weeks for 3 months, followed by
2 mg given as needed
» Macular laser photocoagulation
The primary outcomes were BCVA at 24 weeks and
at 52 weeks, the proportion of eyes that gained 15
letters, and mean change in central foveal thickness.
© 2012 Direct One Communications, Inc. All rights reserved.
46
Aflibercept in DME:
DA VINCI Trial
At 52 weeks, the mean improvements in BCVA were:
+11.0 letters in the group given 0.5 mg of aflibercept
every 4 weeks
+13.1 letters in the group given 2 mg of aflibercept
every 4 weeks
+9.7 letters in the group given 2 mg of aflibercept
every 4 weeks after the initial loading doses
+12 letters in those given 2 mg of aflibercept as
needed after the initial loading doses
+1.3 letters for those who underwent laser therapy.
© 2012 Direct One Communications, Inc. All rights reserved.
47
Aflibercept in DME:
DA VINCI Trial
In addition, > 15 letters of visual acuity were gained by:
40.9% of the group given 0.5 mg of aflibercept every
4 weeks
45.5% of the group given 2 mg of aflibercept every 4
weeks
23.6% of the group given 2 mg of aflibercept every 4
weeks after the initial loading doses
42.2% of those given 2 mg of aflibercept as needed
after the initial loading doses
11.4% of those who underwent laser therapy
© 2012 Direct One Communications, Inc. All rights reserved.
48
Aflibercept in DME:
DA VINCI Trial
The mean reduction in central foveal thickness was:
165.4 µm in the group given 0.5 mg every 4 weeks
227.4 µm in the group given 2 mg every 4 weeks
187.8 µm in the group given 2 mg every 4 weeks after
the initial loading doses
180.3 µm in those given 2 mg as needed after the
initial loading doses
58.4 µm for those who underwent laser therapy
No significant ocular or systemic safety signals were
identified.
© 2012 Direct One Communications, Inc. All rights reserved.
49
Aflibercept in DME:
DA VINCI Trial
Aflibercept therapy has resulted in promising results
when compared with laser monotherapy for treating
patients with DME.
Future randomized trials are needed to evaluate the
safety and long-term efficacy of the approved 2-mg
dose of the drug in treating DME.
Given the abundance of efficacy data reported for
ranibizumab therapy, a head-to-head comparison of
aflibercept with ranibizumab monotherapy would
provide valuable information for clinicians treating
patients with DME.
© 2012 Direct One Communications, Inc. All rights reserved.
50
Conclusion
© 2012 Direct One Communications, Inc. All rights reserved.
51
A Place in DME Therapy
Focal/grid macular laser monotherapy, a DME
therapy that has been used for more than 20 years,
now may not represent best clinical practice.
Enough separate and repeated Level 1 evidence has
shown treatment with VEGF inhibitors, and
especially ranibizumab, to improve visual acuity and
reduce central foveal thickness, as assessed by OCT,
in affected patients.
Thus, ranibizumab, which is now approved by the
FDA for use in the treatment of DME, should be a
part of regular DME management.
© 2012 Direct One Communications, Inc. All rights reserved.
52
Evidence from Clinical Trials
Results of both the RESTORE and REVEAL trials
showed that ranibizumab used alone and with laser
therapy produce comparable results.
The addition of laser therapy did not significantly
decrease the treatment burden when compared with
ranibizumab monotherapy.
The best VEGF-inhibitor administration protocol—
monthly injections, as-needed treatment, or a treatand-extend paradigm—has yet to be established.
© 2012 Direct One Communications, Inc. All rights reserved.
53
Ranibizumab Monotherapy in DME
Results from both the RISE and RIDE trials provide
evidence that monthly injections of ranibizumab
alone are safe and effective against DME through at
least 24 months of treatment.
In the trials reported, ranibizumab monotherapy
ultimately resulted in lower progression to
proliferative retinopathy than did treatment that
included sham laser therapy.
When coupled with the low systemic and ocular
adverse event rate related to ranibizumab use, these
findings appear to support the use of ranibizumab
monotherapy as first-line DME therapy.
© 2012 Direct One Communications, Inc. All rights reserved.
54
Putting Results in Context
Data regarding HbA1c levels and the efficacy of antiVEGF therapy must be evaluated further.
Although the study described was retrospective and
small, it demonstrated a declining efficacy of
bevacizumab with higher HbA1c levels.
Perhaps anti-VEGF monotherapy for DME may not
be as effective in the presence of less optimized
glycemic control in a given patient.
© 2012 Direct One Communications, Inc. All rights reserved.
55
Putting Results in Context
Since the majority of the RISE and RIDE cohorts had
HbA1c levels < 8.0, the results of RISE and RIDE and
the push toward recommending ranibizumab
monotherapy as first-line therapy for DME may need
to kept in perspective.
The evidence for using a higher dose of ranibizumab
was limited in terms of the size of the cohort and the
length of follow-up.
Maximum efficacy seemed to occur at the standard
0.5-mg dose widely used to treat exudative AMD.
© 2012 Direct One Communications, Inc. All rights reserved.
56
Putting Results in Context
SD-OCT may detect DME with a greater sensitivity
than does TD-OCT.
Whether this difference is important, or not, to the
ultimate clinical outcome still must be established.
© 2012 Direct One Communications, Inc. All rights reserved.
57
Treatment Alternatives for DME
The dexamethasone intravitreal implant may
provide an additional complement in cases of
refractory DME, based, so far, on little evidence.
The role of aflibercept in treating DME remains
unclear.
Initial phase II data demonstrate promising efficacy
and safety with the use of these agents.
© 2012 Direct One Communications, Inc. All rights reserved.
58
Peering into the Future
The results of these trials likely will result in more
questions and numerous research studies.
They stress that treatment with focal/grid macular
laser alone no longer is the only standard of care to
treat DME.
As the results of the ANCHOR and MARINA trials in
2006 shifted the therapeutic paradigm for
neovascular AMD, the landscape of approaches to
DME is changing also.
© 2012 Direct One Communications, Inc. All rights reserved.
59
References
1.
Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy: IV. diabetic
macular edema. Ophthalmology. 1984; 91:1464–1474.
2.
Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology.
1998;105:998–1003.
3.
International Diabetes Federation. IDF Diabetes Atlas. 4th ed. Brussels, Belgium: IDF Executive Office;
2009. http://www.diabetesatlas.org/. Accessed June 4, 2012.
4.
Javitt JC, Aiello LP, Chiang Y, et al. Preventive eye care in people with diabetes is cost-saving to the federal
government: implications for health-care reform. Diabetes Care. 1994;17:909–917.
5.
Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular
edema: Early Treatment Diabetic Retinopathy Study reports number 1. Arch Ophthalmol. 1985;103:1796–
1806.
6.
Diabetic Retinopathy Clinical Research Network; Elman MJ, Aiello LP, et al. Randomized trial evaluating
ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema.
Ophthalmology. 2010;117:1064–1077.
7.
Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or
combined with laser versus laser monotherapy for diabetic macular edema. RESTORE Study Group.
Ophthalmology. 2011; 118:615–625.
8.
Rusu I, Wong SH, Barbazetto I. The effect of hemoglobin A1c on bevacizumab response in patients with
diabetic macular edema. Presented at the 2012 Annual Meeting of the Association for Research in Vision
and Ophthalmology (ARVO); May 6–10, 2012; Fort Lauderdale, Florida. Abstract 415.
9.
Karth PA, Chang A, Wiroskto W. Predicting the response to intravitreal anti-VEGF based on the response
in the fellow eye in clinically significant diabetic macular edema. Presented at the 2012 Annual Meeting of
the Association for Research in Vision and Ophthalmology (ARVO); May 6–10, 2012; Fort Lauderdale,
Florida. Abstract 4666.
© 2012 Direct One Communications, Inc. All rights reserved.
60
References
10. Cunha-Vaz J, Faria de Abreu JR, Campos AJ. Early breakdown of the blood-retinal barrier in diabetes. Br J
Ophthalmol. 1975;59:649–656.
11. Qaum T, Xu Q, Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest
Ophthalmol Vis Sci. 2001;42:2408–2413.
12. Ip MS, Domalpally A, Wong P, Hopkins JJ, Ehrlich JS. Long-term effects of intravitreal ranibizumab (RBZ)
on diabetic retinopathy severity and progression. Presented at the 2012 Annual Meeting of the Association
for Research in Vision and Ophthalmology (ARVO); May 6–10, 2012; Fort Lauderdale, Florida. Abstract
1336.
13. Nguyen Q, Brown D, Marcus D. Ranibizumab for diabetic macular edema results from 2 phase III
randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801.
14. Turpcu A, Colman S, Suner IJ, et al. The responsiveness of the National Eye Institute Visual Function
Questionniare-25 (NEI VFQ-25) to visual acuity gains in diabetic macular edema patients. Presented at the
2012 Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO); May 6–10,
2012; Fort Lauderdale, Florida. Abstract 6346.
15. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related
macular degeneration. N Engl J Med. 2006;355:1432–1444.
16. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration.
N Engl J Med. 2006;355:1419–1431.
17. Varma R, Bressler NM, Doan Q, et al. Cases of legal blindness and visual impairment avoided using
ranibizumab for diabetic macular edema in the United States. Presented at the 2012 Annual Meeting of the
Association for Research in Vision and Ophthalmology (ARVO); May 6–10, 2012; Fort Lauderdale, Florida.
Abstract 5739.
© 2012 Direct One Communications, Inc. All rights reserved.
61
References
18. Ferrone P, Jonisch J. Ranibizumab dose and treatment interval comparison for the treatment of diabetic
macular edema: final two-year results. Presented at the 2012 Annual Meeting of the Association for
Research in Vision and Ophthalmology (ARVO); May 6–10, 2012; Fort Lauderdale, Florida. Abstract 1335.
19. Do DV, Campochiaro PA, Boyer DS, et al; READ Study Group. 6 Month results of the READ 3 study:
ranibizumab for edema of the macula in diabetes. Presented at the 2012 Annual Meeting of the Association
for Research in Vision and Ophthalmology (ARVO); May 6–10, 2012; Fort Lauderdale, Florida. Abstract
5282.
20. Khwaja AA, Agbedia O, Sepah YJ, et al; READ-3 Study Group. Comparison of treatment of fellow eyes
treated with ranibizumab with study eye of patients enrolled in the READ-3 study. Presented at the 2012
Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO); May 6–10, 2012;
Fort Lauderdale, Florida. Abstract 4668.
21. Aktar A. Detection and retreatment of diabetic macular edema in the READ-3 study using time-domain and
spectral domain OCT. Presented at the 2012 Annual Meeting of the Association for Research in Vision and
Ophthalmology (ARVO); May 6–10, 2012; Fort Lauderdale, Florida. Abstract 6963.
22. Mitchell P. 2-Year safety and efficacy outcome of ranibizumab 0.5 mg in patients with visual impairment
due to diabetic macular edema: an interim analysis of the RESTORE Extension Study. Presented at the
2012 Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO); May 6–10,
2012; Fort Lauderdale, Florida. Abstract 4667.
23. Ohji M, Ishibashi T Sr; REVEAL Study Group. Efficacy and safety of ranibizumab 0.5 mg as monotherapy
or adjunctive to laser versus laser monotherapy in Asian patients with visual impairment due to diabetic
macular edema: 12-month results of the REVEAL study. Presented at the 2012 Annual Meeting of the
Association for Research in Vision and Ophthalmology (ARVO); May 6–10, 2012; Fort Lauderdale, Florida.
Abstract 4664.
© 2012 Direct One Communications, Inc. All rights reserved.
62
References
24. Zucchiatti I, Lattanzio R, Cascavilla ML, et al. Dexamethasone intravitreal implant in patients with
refractory diabetic macular edema. Presented at the 2012 Annual Meeting of the Association for Research
in Vision and Ophthalmology (ARVO); May 6–10, 2012; Fort Lauderdale, Florida. Abstract 404.
25. Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc
Natl Acad Sci U S A. 2002;99:11393–11398.
26. Rakic JM, Lambert V, Devy L, et al. Placental growth factor, a member of the VEGF family, contributes to
the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3186–3193.
27. Do DV, Nguyen QD, Boyer D, et al. One-year outcomes of the DA VINCI study of VEGF Trap-Eye in eyes
with diabetic macular edema. Ophthalmology. 2012;119:1658–1665.
© 2012 Direct One Communications, Inc. All rights reserved.
63