05-01BindingEnergy
advertisement
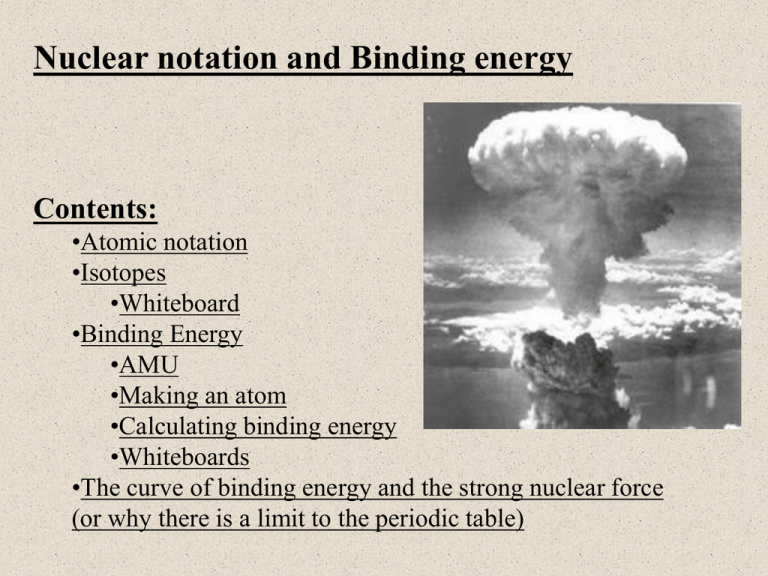
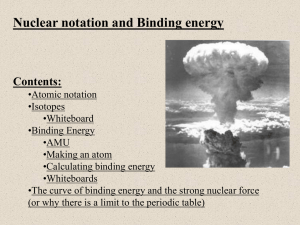
Nuclear notation and Binding energy Contents: •Atomic notation •Isotopes •Whiteboard •Binding Energy •AMU •Making an atom •Calculating binding energy •Whiteboards •The curve of binding energy and the strong nuclear force (or why there is a limit to the periodic table) Atomic notation A Z 12 6 X C X = Symbol (C, Au) A = Atomic Mass Number = #nucleons (Protons + Neutrons) Z = Atomic Number = #protons Carbon A = 12, Z = 6. #neutrons? TOC Isotopes 12 6 14 6 C C Carbon 12 has 6 Neutrons Carbon 14 has 8 Neutrons Carbon 14 is an isotope of Carbon Chemically the same Nuclear-lly different (it’s unstable) C-14, C-12 TOC Whiteboards: Atomic Notation 1|2|3|4|5 TOC What is the Atomic notation for tritium? (tritium is an isotope of Hydrogen with 2 neutrons) Hydrogen has a Z = 1, A = 1 p + 2 n = 3 3/1 H W 10 protons, 12 neutrons. What is its atomic notation? Z = 10, A = 10 + 12 = 22, element 10 is Ne 22/10 Ne W How many neutrons in U 235? (235 = A) (1) U is element 92 235 - 92 = 143 neutrons 143 W How many neutrons in Pb 208? (208 = A) (1) Pb is element 82 208 - 82 = 126 neutrons 126 W How many neutrons in Kr 78? (1) Kr is element 36 78 - 36 = 42 neutrons 42 W Binding energy - the energy to take an atom apart Unified Mass Units: (u) C 12 (neutral atom) = 12.0000000 u (defined) 1 u = 1.6605 x 10-27 kg = 931.5 MeV (show c = 2.998x108 m/s) Electron = .00054858 u (not useful) Proton = 1.007276 u (not useful) H (neutral atom) = 1.007825 u (very useful) Neutron = 1.008665 u (very useful) Binding energy - the energy to take an atom apart H (neutral atom) = 1.007825 u (very useful) Neutron = 1.008665 u (very useful) 1 u = 931.5 MeV To take an atom apart: C 14 has a mass of 14.003242 u (p. 1064 in text) C 14 can be taken apart into: 6 H atoms: 6(1.007825 u) (i.e. 6 p + 6 e) 8 Neutrons: 8(1.008665 u) Total “taken apart” mass = 6(1.007825 u) + 8(1.008665 u) Total “taken apart” mass = 14.116270 u Mass “defect” = 14.11627 u - 14.003242 u = 0.113028 u 0.113028 u - Represents energy needed to take atom apart Binding energy = (0.113028 u)(931.5 MeV/u) = 105.3 MeV TOC Binding energy - the energy to take an atom apart H (neutral atom) = 1.007825 u (very useful) Neutron = 1.008665 u (very useful) 1 u = 931.5 MeV Binding energy in general 1. Look neutral atom mass in Appendix F (p 1064) 2. Break atom into H and n 3. Subtract neutral atom mass from taken apart mass 4. Multiply mass defect by 931.5 Mev/u TOC Whiteboards: Binding Energy 1|2|3 TOC What is the binding energy for Nitrogen 13? (Z = 7) N 13 mass = 13.005738 u H = 1.007825 u Neutron = 1.008665 u 1 u = 931.5 MeV Atom mass = 13.005738 7H + 6n = 7(1.007825 u) +6(1.008665 u) = 13.106765 u Binding energy = (13.106765 - 13.005738)931.5 MeV/u = 94.11 MeV 94.11 MeV W What is the binding energy for Carbon 12? (Z = 6) C 12 mass = 12.000000 u (duh?) H = 1.007825 u Neutron = 1.008665 u 1 u = 931.5 MeV Atom mass = 12.000000 6H + 6n = 6(1.007825 u) +6(1.008665 u) = 12.09894 u Binding energy = (12.098940 – 12.000000)931.5 MeV/u = 92.16 MeV Binding energy per nucleon: C 12: 92.16/12 = 7.68 MeV/nucleon C 14: 105.3/14 = 7.52 MeV/nucleon (less) 92.16 MeV W What is the binding energy for Carbon 10? (Z = 6) C 10 mass = 10.231610 u H = 1.007825 u Neutron = 1.008665 u 1 u = 931.5 MeV Atom mass = 10.231610 6H + 4n = 6(1.007825 u) +4(1.008665 u) = 10.08161 u Binding energy = (10.08161 - 10.23161)931.5 MeV/u = -139.7 MeV (Carbon 10 does not exist) -139.7 W