Units
advertisement

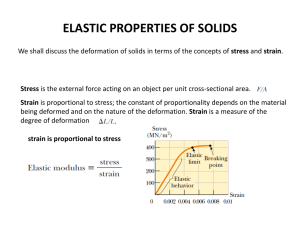
Problem Solving • Chemistry college life is all about solving problems • Chemistry: it makes sense! • Develop a logical plan (series of steps) from your known to your unknowns http://www.geneseo.edu/~mcknight/ Problem Solving and Dimensional Analysis • Many problems in chemistry involve using relationships to convert one unit of measurement to another • Conversion factors are relationships between two units – May be exact or measured • Conversion factors generated from equivalence statements – e.g., 1 inch = 2.54 cm can give 2.54cm or 1in 1in 2.54cm Problem Solving and Dimensional Analysis • Arrange conversion factors so given unit cancels – Arrange conversion factor so given unit is on the bottom of the conversion factor • May “string” conversion factors – So we do not need to know direct relationships, as long as we can find steps that leads to the desired units (known unknown) desired unit given unit desired unit given unit “Must have a Plan” • a conceptual plan is a visual outline that shows the strategic route required to solve a problem • for unit conversion, the plan focuses on units and how to convert one to another • for problems that require equations, the conceptual plan focuses on solving the equation to find an unknown value Concept Plans and Conversion Factors • Convert inches into centimeters 1) Find relationship equivalence: 1 in = 2.54 cm 2) Write concept plan in cm 3) Change equivalence into conversion factors with starting units on the bottom 2.54 cm 1 in A Systematic Approach • Sort the information from the problem – identify the given quantity and unit, the quantity and unit you want to find, any relationships implied in the problem • Design a strategy to solve the problem (roadmap) – Concept plan • sometimes may want to work backwards • each step involves a conversion factor or equation • Apply the steps in the concept plan – check that units cancel properly – multiply terms across the top and divide by each bottom term • Check the answer – double check the set-up to ensure the unit at the end is the one you wished to find – check to see that the size of the number is reasonable • since centimeters are smaller than inches, converting inches to centimeters should result in a larger number Example: Convert 1.76 yd. to centimeters • • • • • Sort information Strategize Follow the concept plan to solve the problem Sig. figs. and round Check Given: Find: Concept Plan: Relationship Solution: 1.76 yd length, cm yd m cm 1.094 yd = 1 m 1 m = 100 cm 1m 100 cm 1.79 yd 160.8775 cm 1.094 yd 1m Round: 160.8775 cm = 161 cm Check: Units & magnitude are correct Practice – Convert 30.0 mL to quarts (1 L = 1.057 qt) (Hint: 1000 mL makes 1 L) Convert 30.0 mL to quarts • • • • • Sort information Strategize Follow the plan to solve the problem Sig. figs. and round Check Given: Find: Concept Plan: Relationship: Solution: 30.0 mL volume, qts mL L qt 1 L = 1.057 qt 1 L = 1000 mL 1L 1.057 qt 30.0 mL 0.03171 qt 1000 mL 1L Round: 0.03171 qt = 0.0317 qt Check: Units & magnitude are correct Problem Solving with Equations • When solving a problem using an equation, you are usually given all the variables except the one you want to find • Solve the equation for the variable you wish to find, then substitute and compute Using Density in Calculations Concept Plans: Mass Density Volume m, V D Mass Volume Density m, D V V, D m Mass Density Volume Density Calculations • We can use density as a conversion factor between mass and volume!! – – density of H2O = 1.0 g/mL \ 1.0 g H2O = 1 mL H2O density of Pb = 11.3 g/cm3 \ 11.3 g Pb = 1 cm3 Pb How much does 4.0 cm3 of lead weigh? 4.0 cm3 Pb x 11.3 g Pb 1 cm3 Pb = 45 g Pb Question: The mass of fuel in a jet must be calculated before each flight to ensure that the jet is not too heavy. A 747 jet is fueled with 173,231 L of jet fuel. If the density of the fuel is 0.738 g/mL, what is the mass of the fuel in kilograms? Example: What is the mass in kg of 173,231 L of jet fuel whose density is 0.738 g/mL? • • • • • Sort information Strategize Follow the concept plan to solve the problem Sig. figs. and round Check Given: Find: Concept Plan: 173,231 L density = 0.738 g/mL mass, kg L mL g kg 1 mL = 0.738 g, 1 mL = 10-3 L Relationship 1 kg = 1000 g Solution: 1 mL 0.738 g 1 kg 173,231 L -3 10 L 1 mL 1000 g 1.33 105 kg Round: 1.3 x 105 kg Check: Units & magnitude are correct Counting Atoms by Moles • If we can find the mass of a particular number of atoms, we can use this information to convert the mass of an element sample into the number of atoms in the sample. The number of atoms we use is 6.022 x 1023 and we call this a mole • – 1 mole = 6.022 x 1023 entities • Like 1 dozen = 12 entities Avogadro’s Number Example: Calculate the number of atoms in 2.45 mol of copper Given: Find: Concept Plan: 2.45 mol Cu atoms Cu mol Cu atoms Cu 6.0221023 atoms 1 mol 1 mol = 6.022 x 1023 atoms Solution: 6.0221023 atoms 2.45 molCu 1 mol 1.481024 atomsCu Check: since the number is slightly greater than twice Avogadro’s number, it make sense Relationship Between Moles and Mass • • • • The mass of one mole of atoms is called the molar mass The molar mass of an element, in grams, is numerically equal to the element’s atomic mass, in amu The lighter the atom, the less a mole weighs The lighter the atom, the more atoms there are in 1 g Mole and Mass Relationships hydrogen carbon Weight of Pieces in 1 atom 1 mole 1.008 amu 6.022 x 1023 atoms 12.01 amu 6.022 x 1023 atoms Weight of 1 mole 1.008 g 12.01 g oxygen 16.00 amu 6.022 x 1023 atoms 16.00 g sulfur 32.06 amu 6.022 x 1023 atoms 32.06 g calcium 40.08 amu 6.022 x 1023 atoms 40.08 g chlorine 35.45 amu 6.022 x 1023 atoms 35.45 g copper 63.55 amu 6.022 x 1023 atoms 63.55 g Substance 1 mole sulfur 32.06 g 1 mole carbon 12.01 g Example: Calculate the # moles of carbon in 0.0265 g of pencil “lead” Given: Find: Concept Plan: 0.0265 g C mol C gC mol C 1 mol 12.01g 1 mol C = 12.01 g Solution: 1 mol 0.0265g C 12.01g 2.2110-3 molC Check: since the given amount is much less than 1 mol C, the number makes sense Example: How many copper atoms are in a copper penny weighing 3.10 g? Given: Find: Concept Plan: Solution: 3.10 g Cu atoms Cu g Cu mol Cu 1 mol 63.55g 1 mol Cu = 63.55 g, 1 mol = 6.022 x 1023 atoms Cu 6.0221023 atoms 1 mol 1 molCu 6.0221023 at oms 3.10 g Cu 63.55g Cu 1 mol 2.94 1022 at omsCu Check: since the given amount is much less than 1 mol Cu, the number makes sense