Chapter 6 Lecture
advertisement

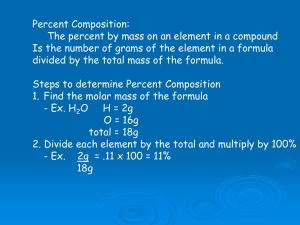
Chapter 6 Chemical Composition 2006, Prentice Hall CHAPTER OUTLINE The Mole Concept Molecular & Molar Mass Calculations Using the Mole Percent Composition Chemical Formulas Calculating Empirical Formulas Molecular Formulas 2 Why is Knowledge of Composition Important? • everything in nature is either chemically or physically combined with other substances • to know the amount of a material in a sample, you need to know what fraction of the sample it is • Some Applications: – – – – the amount of sodium in sodium chloride for diet the amount of iron in iron ore for steel production the amount of hydrogen in water for hydrogen fuel the amount of chlorine in freon to estimate ozone depletion Tro's Introductory Chemistry, Chapter 6 THE MOLE CONCEPT Chemists find it more convenient to use mass relationships in the laboratory, while chemical reactions depend on the number of atoms present. In order to relate the mass and number of atoms, chemists use the SI unit mole (abbreviated mol). 4 THE MOLE CONCEPT The number of particles in a mole is called Avogadro’s number and is 6.02x1023. 1 mole equals to 6.02 x 1023 Avogadro’s number (NA) 5 THE MOLE CONCEPT A mole is a very large quantity 6.02x1023 If 10,000 people started to count Avogadro’s number and counted at the rate of 100 numbers per minute each minute of the day, it would take over 1 trillion years to count the total number. 6 THE MOLE CONCEPT 1 mole H atoms = 6.02x1023 H atoms 1 mole H2 molecules = 6.02x1023 H2 molecules = 2 x (6.02x1023) H atoms 1 mole H2O molecules = 6.02x1023 H2O molecules = 2 x (6.02x1023) H atoms = 6.02x1023 O atoms 1 mole Na+ ions = 6.02x1023 Na+ ions 7 THE MOLE CONCEPT The atomic mass of one atom expressed in amu is numerically the same as the mass of 1 mole of atoms of the element expressed in grams. Mass Mass Massof of of111H Mg Clatom atom atom = =1.008 = 35.45 24.31 amu amu amu Mass Mass Massof of of111mol mol molHMg Cl atoms atoms atoms == 1.008 = 35.45 24.31 grams gg 8 MOLE DAY Chemists and chemistry students celebrate two days in the year in honor of the Mole and call them Mole Days. October 23rd Jun 2nd 6:02 a.m. 10:23 a.m. 9 MOLECULAR MASS The sum of atomic masses of all the atoms in one molecule of a substance is called molecular mass, and is measured in amu. Mass of one molecule of H2O 2 H atoms = 2 (1.008 amu) = 2.016 amu 1 O atom = 1 (16.00 amu) = 16.00 amu Molecular mass 18.02 amu 10 Relationship Between Moles and Mass • The mass of one mole of atoms is called the molar mass • The molar mass of an element, in grams, is numerically equal to the element’s atomic mass, in amu MOLAR MASS The mass of one mole of a substance is called molar mass, and is measured in grams. Mass of one mole of H2O 2 mol H atoms = 2 (1.008 g) = 2.016 g 1 mol O atom = 1 (16.00 g) = 16.00 g Molar mass 18.02 g 12 CALCULATIONS USING THE MOLE Conversions between mass, mole and particles can be done using molar mass and Avogadro’s number. Avogadro’s Molar number mass Mass of a substance MM Moles of a substance NA Particles of a substance 13 Example 1: What is the mass of 5.00 mol of water? 18.02 g 5.00 m ol H 2 O x = 90.1 g H 2 O 1 m ol 3 significant figures Molar mass 14 Example 2: How many Mg atoms are present in 5.00 g of Mg? mass mol atoms 5.00 g M g x Molar mass 1 m ol 24.3 g x 6.02 x 1023 1 atom s = m ol 1.24x1023 atoms Mg Avogadro’s 3 significant figures number 15 Example 3: How many molecules of HCl are present in 25.0 g of HCl? mass mol molecules 25.0 g H C l x 1 m ol 36.45 g x 6.02 x 1023 m olecu les 1 = m ol 4.13 x 1023 molecules HCl 3 significant figures 16 PERCENT COMPOSITION The percent composition of a compound is the mass percent of each element in the compound. M ass % X = (n o. of X in form u la) x (m olar m ass of X ) x 100 m olar m ass of com p ou n d Total mass of element Total mass of compound 17 Example 1: Calculate the percent composition of sodium chloride (NaCl). Step 1: determine molar mass of NaCl 1 mol Na atoms 1 (22.99 g) = 22.99 = 1 g (35.45 g) = 35.45 g 1 mol Cl atom = Molar 58.44 mass g/mol 18 Example 1: Step 2: calculate the mass % of each element 22.99 g % Na = 58.44 g 35.45 g % Cl = x 100 = 39.34% x 100 = 60.66% 58.44 g Sum = 100% 19 Example 2: 1.63 g of zinc combines with 0.40 g of oxygen to form zinc oxide. Determine the % composition of the compound formed. Step 1: determine total mass of sample mass of sample = 1.63 g + 0.40 g = 2.03 g 20 Example 2: Step 2: calculate the mass % of each element % Zn = %O= 1.63 g x 100 = 80.3% 2.03 g 0.40 g x 100 = 19.7% 2.03 g Sum = 100% 21 Example 3: Calculate the percent composition of sodium hydroxide (NaOH). Step 1: determine molar mass of NaOH 1 mol Na atoms = 1 (23.0 g) = 23.0 g 1 mol O atom = 1 (16.0 g) = 16.0 g 1 mol H atoms = 1 (1.01 g) = 1.01 g Molar mass 40.0 g/mol 22 Example 3: Calculate the percent composition of sodium hydroxide (NaOH). Step 1: determine molar mass of NaOH 1 mol Na atoms = 1 (23.0 g) = 23.0 g 1 mol O atom = 1 (16.0 g) = 16.0 g 1 mol H atoms = 1 (1.01 g) = 1.01 g Molar mass 40.0 g/mol 23 CHEMICAL FORMULAS Molecular formula Shows Can be the written actual for number molecular of atoms compounds in a compound only Empirical formula Shows simplest Can bethe written for ratio of atoms in a molecular and compound ionic compounds 24 CHEMICAL FORMULAS Molecular Empirical Multiplier H2O2 HO 2 H2O H2O 1 C6H12O6 CH2O 6 C6H6 CH 6 C2H2 CH 2 C6H12 CH2 6 25 CHEMICAL FORMULAS Several compounds may possess the same percent composition and empirical formula, but different molecular formulas. Formula CH Composition %C %H 92.3 7.7 Molar mass (g/mol) 13.02 C2H2 92.3 7.7 26.04 (2x13.02) C6H6 92.3 7.7 78.12 (6x13.02) 26 Finding an Empirical Formula 1) convert the percentages to grams a) skip if already grams 2) convert grams to moles a) use molar mass of each element 3) write a pseudoformula using moles as subscripts 4) divide all by smallest number of moles 5) multiply all mole ratios by number to make all whole numbers a) if ratio ?.5, multiply all by 2; if ratio ?.33 or ?.67, multiply all by 3, etc. b) skip if already whole numbers If, after dividing by the smallest number of moles, the subscripts are not whole numbers, multiply all the subscripts by a small whole number to arrive at wholenumber subscripts. CALCULATING EMPIRICAL FORMULAS Arsenic (As) reacts with oxygen (O) to form a compound that is 75.7% As and 24.3% oxygen by mass. What is the empirical formula for this compound? Step 1: Percent to mass Assume 100 g 75.7% As 75.7 g As 24.3% O 24.3 g O 29 CALCULATING EMPIRICAL FORMULAS Step 2: Mass to mole 75.7 g As x 24.3 g O x 1 m ol = 1.01 m ol A s 74.9 g 1 m ol = 1.52 m ol O 16.0 g Use atomic mass of oxygen 30 CALCULATING EMPIRICAL FORMULAS Step 3: Divide by small As = 1.01 m ol = 1.00 1.01 m ol O= 1.52 m ol = 1.50 1.01 m ol 31 CALCULATING EMPIRICAL FORMULAS Step 4: Multiply till Whole AsAs 1.002O3 1.50 x2=2 x2=3 32 Example 1: Determine the empirical formula for a compound containing 11.2% H and 88.8% O. mol H = mol O = 11.2 g x 1 m ol = 11.1 m ol H 1.01 g 88.8 g x 1 m ol 11.1 = 2.0 5.55 = 5.55 m ol O 16.0 g 5.55 = 1.0 5.55 H2O 33 Example 2: Determine the empirical formula for a compound with the following percent 52.14% C, C2Hcomposition: O 6 13.12% H, 34.73% O. mol C = 52.14 g x mol H = 13.12 mol O = g x 34.73 g x 1 m ol = 4.345 m ol C 4.345 12.0 g 2.17 1 m ol 13.0 = 13.0 m ol H 1.01 g 2.17 1 m ol 2.17 16.0 g = 2.17 m ol O = 2.0 = 6.0 = 1.0 2.17 34 MOLECULAR FORMULAS Molecular formula can be calculated from empirical formula if molar mass is known. Molecular formula = (empirical formula) n n (m ultiplier) = M olar m ass M ass of em pirical form ula 35 Example 1: A compound of N and O with a molar mass of 92.0 g, has the empirical formula of NO2. What is its molecular formula? Mass of empirical formula = 14.0 + 2(16.0) = 46.0 n = 92.0 g = 2 46.0 g Molecular formula = 2 x (NO2) = N2O4 36 Example 2: Calculate the empirical and molecular formulas of a compound that contains 80.0% C and 20.0% H, and has a molar mass of 30.00 g. mol C = mol H = 80.0 g x 20.0 g x Empirical formula 1 m ol = 6.667 m ol C 6.667 12.0 g 6.667 1 m ol 19.8 = 19.8 m ol H 1.01 g = 1.0 = 3.0 6.667 CH3 37 Example 2: Empirical formula = CH3 Mass of empirical formula = 12.0 + 3(1.01) = 15.0 n = 30.0 g = 2 15.0 g Molecular formula = 2 x (CH3) = C2H6 38 THE END 39