Units - Chemistry notes
advertisement

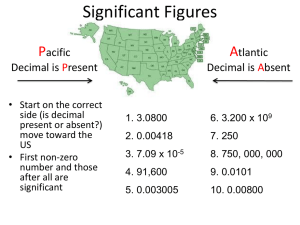
Chapter 3: Measurement CMH 101 Luca Preziati Measurement DEF = Quantitative observation • Comparison to an agreed upon standard • Every measurement has a number and a unit Scientists have measured the average global temperature rise over the past century to be The number tells you 1.what multiple of the standard the object measures 2.the uncertainty in the measurement 0.6 °C The unit tells you what standard you are comparing your object to Chapter 3: Measurement Scientific Notation is a way of writing large and small numbers The sun’s diameter is 1,392,000,000 m The sun’s diameter is 1.392 x 109 m An atom’s average diameter is 0.000 000 000 3 m An atom’s average diameter is 3 x 10-10 m Large Number = Positive Exponent Small Number = Negative Exponent 1.392 x 109 m 3 x 10-10 m Chapter 3: Measurement Writing a number in scientific notation 1,392,000,000 m 1. Locate the decimal point 2. Move the decimal point until a number between 1 and 10 is obtained 3. Multiply the new number by 10n 4. n is the number of places you moved the decimal point 5. Large number? n is positive Small number? n is negative . 1,392,000,000 m 1.392,000,000. m 1.392 x 10 ? 9 m 1.392 x 10 9 m 3.5 : Significant Figures (Pg. 69) Significant Figures Writing Numbers to Reflect Uncertainty Exact Values • Can be obtained by counting or by definition • Exact values have “unlimited (∞) significant figures” Measurements • Are obtained from instruments • The number of significant figures reflects the instrument Uncertainty. All the digits written are known with certainty except the last one, which is an estimate 1.2 grams Certain Estimated (Doubtful) 3.5 : Significant Figures (Pg. 69) Counting Significant Figures 1. Write the number in scientific notation 2. Count the number of figures 0.000 000 000 35 m 3.5 x 10-10 m 2 Significant figures Note: Zeros at the end of a number without a written decimal point are ambiguous and should be avoided by using scientific notation. if 150 has 2 sig. figs. then 1.5 x 102 but if 150 has 3 sig. figs. then 1.50 x 102 3.5 : Significant Figures (Pg. 69) Counting Significant Figures 1: Non zero digits are always significant. Thus 6.23 km has three significant figures. 2: Zeros in the middle of a figure are always significant. Thus 56.0309 g has 6 significant figures. 3: Zeros at the beginning of a number are only decimal place holders; they are not significant. Thus 0.00928 cm has three significant figures. 4: Zeros at the end of a number but after the decimal point are significant. Thus 21.30 mL has four significant figures. 5: Zeros at the end of a number but before the decimal point are ambiguous. Thus 21,000 kg has 2 significant figures, but 21,000.0 has 6 significant figures (rule 4). 3.5 : Significant Figures (Pg. 69) Counting Significant Figures. Examples. How many significant figures are in each of the following numbers? 0.0035 1.080 2371 2.97 × 105 1 dozen = 12 100,000 3.5 : Significant Figures (Pg. 69) +- Addition and Subtraction with Significant Figures When adding or subtracting measurements with significant figures, the result has the same number of decimal places as the measurement with the fewest number of decimal places 5.74 + 2 dec. pl. 4.8 1 dec. pl 0.823 + 3 dec. pl. 3.965 3 dec. pl. 2.651 = 9.214 = 9.21 3 dec. pl. = 0.835 = 2 dec. pl. 0.8 1 dec. pl. 3.5 : Significant Figures (Pg. 69) × ÷ Multiplication and Division with Significant Figures When multiplying or dividing measurements with significant figures, the result has the same number of significant figures as the measurement with the fewest number of significant figures 5.02 × 3 sig. figs. 89,665 × 5 sig. figs. 5.892 ÷ 4 sig. figs. 0.10 = 45.0118 = 45 2 sig. figs. 2 sig. figs. 6.10 = 0.96590 = 0.966 3 sig. figs. 3 sig. figs. 3.4 : Units (Pg. 63) The Standard Units Scientists have agreed on a set of international standard units for comparing all our measurements called the SI units Système International = International System Quantity Unit Symbol length meter m mass kilogram kg time second s kelvin K temperature 3.4 : Units (Pg. 63) Related Units in the SI System • All units in the SI system are related to the standard unit by a power of 10 • The power of 10 is indicated by a prefix • The prefixes are always the same, regardless of the standard unit 3.4 : Units (Pg. 63) Common Prefixes in the SI System (pg.65) Prefix Symbol Decimal Equivalent Power of 10 1,000,000 Base x 106 1,000 Base x 103 mega- M kilo- k deci- d 0.1 Base x 10-1 centi- c 0.01 Base x 10-2 milli- m 0.001 Base x 10-3 micro- m or mc 0.000 001 Base x 10-6 nano- n 0.000 000 001 Base x 10-9 3.4 : Units (Pg. 63) Mass • = Measure of the amount of matter present in an object • SI unit = kilogram (kg) about 2 lbs. 3 oz. • Commonly measure mass in grams (g) or milligrams (mg) 1 kg = 2.2046 pounds, 1 lbs. = 453.59 g 1 kg = 1000 g = 103 g, 1 g = 1000 mg = 103 mg 1 g = 0.001 kg = 10-3 kg, 1 mg = 0.001 g = 10-3 g DEF 3.4 : Units (Pg. 63) Length • = Measure of the two-dimensional distance an object covers • SI unit = meter About 3½ inches longer than a yard • 1 meter = one ten-millionth the distance from the North Pole to the Equator = distance between marks on standard metal rod in a Paris vault = distance covered by a certain number of wavelengths of a special color of light • Commonly use centimeters (cm) 1 m = 100 cm 1 cm = 0.01 m = 10 mm 1 inch = 2.54 cm (exactly) DEF 3.4 : Units (Pg. 63) Volume • = Measure of the amount of three-dimensional space occupied • SI unit = cubic meter (m3) a Derived Unit • Commonly measure solid volume in cubic centimeters (cm3) 1 m3 = 106 cm3 1 cm3 = 10-6 m3 = 0.000001 m3 • Commonly measure liquid or gas volume in milliliters (mL) 1 L is slightly larger than 1 quart 1 L = 1 dL3 = 1000 mL = 103 mL 1 mL = 0.001 L = 10-3 L 1 mL = 1 cm3 DEF 3.6 : Metric-USCS Conversions (Pg. 77) Common Units and Their Equivalents Length 1 kilometer (km) = 0.6214 mile (mi) 1 meter (m) = 39.37 inches (in.) 1 meter (m) = 1.094 yards (yd) 1 foot (ft) = 30.48 centimeters (cm) 1 inch (in.) = 2.54 centimeters (cm) exactly 1 kilogram (kg) = 2.205 pounds (lb) 1 pound (lb) = 453.59 grams (g) 1 ounce (oz) = 28.35 (g) Mass 3.6 : Metric-USCS Conversions (Pg. 77) Common Units and Their Equivalents Volume 1 liter (L) = 1000 milliliters (mL) 1 liter (L) = 1000 cubic centimeters (cm3) 1 liter (L) = 1.057 quarts (qt) 1 U.S. gallon (gal) = 3.785 liters (L) 3.3 : Dimensional Analysis (Pg. 56) Problem Solving and Dimensional Analysis • Arrange conversion factors so starting unit cancels Arrange conversion factor so starting unit is on the bottom of the conversion factor • May string conversion factors So we do not need to know every relationship, as long as we can find something else the beginning and ending units are related to unit 1 = unit 2 unit 1 x unit 2 unit 1 = unit 2 3.3 : Dimensional Analysis (Pg. 56) Dimensional Analysis Example 1: Convert 7.8 km to miles 1. Write down the Given quantity and its unit Given: 7.8 km (unit 1) 2. Write down the quantity you want to Find and unit Wanted: ? Miles (unit 2) 3. Write down the appropriate Conversion Factors Conversion Factors: 4. Rearrange the conversion Factor Solution: 1 km = 0.6214 mi 7.8 km 0.6214 mi 4.84692 mi 1 km 5. Sig. Figs. and Round Round: 4.84692 mi = 4.8 mi 6. Check Check: Units & Magnitude are correct 3.3 : Dimensional Analysis (Pg. 56) Dimensional Analysis Example 2: Convert 20.0 lbs to kg 1. Write down the Given quantity and its unit Given: 20.0 lbs (unit 1) 2. Write down the quantity you want to Find and unit Wanted: ? kg (unit 2) 3. Write down the appropriate Conversion Factors Conversion Factors: 4. Rearrange the conversion Factor Solution: 1 kg = 2.205 lbs 20 lbs 1 kg 9.07029 kg 2.205 lbs 5. Sig. Figs. and Round Round: 9.07029 kg = 9.07 kg 6. Check Check: Units & Magnitude are correct 3.3 : Dimensional Analysis (Pg. 56) Dimensional Analysis Example 3: How many milliliters in 1.00 qt? 1. Write down the Given quantity and its unit Given: 1.00 qt (unit 1) 2. Write down the quantity you want to Find and unit Wanted: ? mL (unit 2) 3. Write down the appropriate Conversion Factors Conversio n Factors: 5. Rearrange the conversion Factor Solution: 1 L = 1.057 qt 1 L = 1000 mL 1.00qt x __1 L__ = 0.94607 L 1.057 qt 0.94607L x 1000 mL = 946.07mL 1L 6. Sig. Figs. and Round Round: 946.07 mL = 946 mL 7. Check Check: Units/Magnitude correct 3.7 : Temperature (Pg. 81) Temperature • • DEF = measure of the average kinetic energy of the molecules in a sample SI unit = Celsius (°C) Water Fahrenheit Celsius Kelvin Freezing point 32 0 273 Boiling Point 212 100 373 C F - 32 1.8 K C 273.15 3.8 : Density (Pg. 84) Volume vs Mass of Brass y = 8.38 x 160 140 120 Mass, g 100 80 60 40 20 0 0.0 2.0 4.0 6.0 8.0 10.0 Volume, cm3 12.0 14.0 16.0 18.0 3.8 : Density (Pg. 84) Ratio of mass:volume Density Mass Volume Solids = g/cm3 1 cm3 = 1 mL Liquids = g/mL Gases = g/L Volume Mass Density Mass Density Volume Volume of a solid can be determined by water displacement Density : solids > liquids >>> gases except ice is less dense than liquid water! 3.8 : Density (Pg. 84) • For equal volumes, denser object has larger mass • For equal masses, denser object has smaller volume • Heating objects causes objects to expand does not effect their mass!! How would heating an object effect its density? • In a heterogeneous mixture, the denser object sinks Why do hot air balloons rise? 3.10 : Dimensional Analysis vs. Algebra (Pg. 88) Density (and Temperature) use equations instead of conversion factors • Example 1: the gasoline in an automobile gas tank has a mass of 60.0 kg and a density of 0.752 g/cm3. What is the volume? • Given: mass = 60.0 kg Density = 0.752 grams/cm3 • Wanted: Volume in L • Conversion Factors: 1000 grams = 1 kg • Example 2: A 55.9 kg person displaces 57.2 L of water when submerged in a water tank. What is the density of the person in g/cm3