Chemistry Worksheet: Sig Figs, Conversions, Density
advertisement
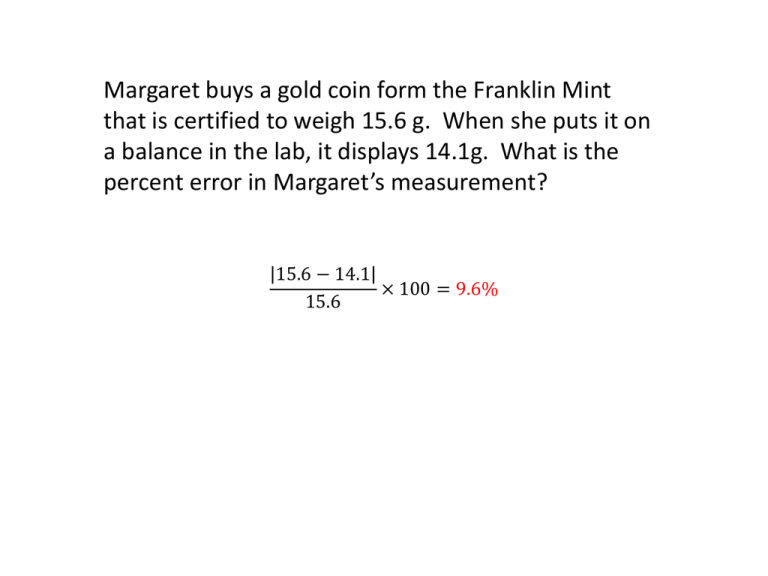
Margaret buys a gold coin form the Franklin Mint that is certified to weigh 15.6 g. When she puts it on a balance in the lab, it displays 14.1g. What is the percent error in Margaret’s measurement? 15.6 − 14.1 × 100 = 9.6% 15.6 Round the following numbers to the number of significant figures indicated in parentheses. 0.002876 (2)= 598 (2)= 102670 (3)= 6.75 x 10-3 (1)= 0.0029 6.0x102 103000 7x10-3 Divide the following numbers and report your answers to the correct number of significant figures in scientific notation. a. b. (4.0x10-6)/(8.0x10-3) = 0.5x10-3 15.0/3.0 = 5.0 5.0x10-4 Subtract the following numbers and report your answers to the correct number of sig figs: a. 1825-70 = 1755 1860 b. 5.0x106-2x105= 4.8 x 106 5x106 Add the following numbers and report the answers to the correct number of sig figs. 19060 + 285=19345 19350 1.2x108 + 5.4x107=1.74x108 1.7 x 108 Match the measurements with the correct SI unit: 1. Length F 2. Mass B 3. Temperature H 4. Time D A. B. C. D. E. Liter Kilogram Celcius Second Kilometer F. Meter G. Cubic Centimeter H. Kelvin I. Millisecond J. Gram Convert 3820dg to ounces using the following conversion factors and the factor label method of analysis. 2.2lbs=1kg, 1000g=1kg, 10dg=1g, 16oz=1lb ****Remember Sig Figs**** 3820𝑑𝑔 1𝑔 10𝑑𝑔 1𝑘𝑔 1000𝑔 2.2𝑙𝑏 1𝑘𝑔 160𝑧 1𝑙𝑏 = 13.4464 13.4𝑜𝑧 How many significant digits are in the following numbers? 10200040 0.00654040 125.0000 1330.0650 0.010001 7 6 7 8 5 What is the density of the following substance? change in y over the change in x = 10𝑔 14𝑚𝑙 = 0.71g/ml Is the following substance: a. Rubidium=1.532g/ml b. Potassium = 0.71g/ml c. Beryllium=1.85g/ml What is the value of 34oC in Kelvin? K=C+273 K=34+273=307K Multiply the following numbers and report the answers in scientific notation to the correct number of sig figs. (4.2x106)(3x10-3) = 12.6 x 109 81 x 9.00= 729 730 1.26 x 1010 What happens to the density of an object when volume decreases? (such as what happens to a balloon on a cold day) Density Increases Write the following numbers in correct scientific notation form. 0.006420 3050728 101.28 x 102 6.420x10-3 3.050728x 106 1.0128x104 Determine the density of this object and report your answer to the correct number of sig figs. 60 50 𝐷= 𝑀 𝑉 4.6𝑔 56.0−52.6 = 1.3𝑔/𝑚𝐿 Calculate the volume of this object in cm3 and then convert to mm3 (report your answers to the correct number of sig figs) I don’t remember the exact measurements, but…. V= L x W x H so… 6.42𝑐𝑚 × 2.85𝑐𝑚 × 4.30𝑐𝑚 = 78.6771 𝑐𝑚3 10𝑚𝑚 3 3 3 78.7𝑐𝑚 =78700mm 1𝑐𝑚 78.7cm3 Rewrite the following equation to solve for mass and then for volume. (2 equations) Which of the following are derived measurements? a. Mass b. Length c. Volume d. Time e. Temperature f. Density Match the equivalent values: 1. 2. 3. 4. 1 kg 1 dag 1 hg 1 mg a. 0.1 kg b. 10000mg c. 0.0001 dag d. 10 hg Do the following multidimensional conversion using the factor label method. Convert 0.63lbs/qt→cg/mL (2.2lbs=1kg, 1.1qt=1L) ****Remember Sig Figs**** 0.63 𝑙𝑏𝑠 1𝑘𝑔 𝑞𝑡 2.2𝑙𝑏𝑠 1000𝑔 1𝑘𝑔 100𝑐𝑔 1𝑔 1.1𝑞𝑡 1𝐿 1𝐿 𝑐𝑔 = 31.5 → 32 1000𝑚𝐿 𝑚𝐿 How would you describe this archer’s skill in terms of accuracy and precision? Precise, but not accurate











