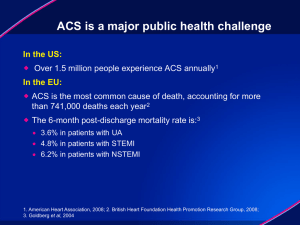
C. Michael Gibson LBCT AHA 2011
advertisement

C. Michael Gibson, M.S., M.D., Jessica Mega, M.P.H., M.D., & Eugene Braunwald, M.D. on behalf of the ATLAS ACS 2 TIMI 51 Investigators Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome – Thrombolysis in Myocardial Infarction 51 Trial (ATLAS-ACS 2 TIMI 51): A Randomized, Double-Blind, Placebo Controlled Study to Evaluate the Efficacy and Safety of Rivaroxaban in Subjects with Acute Coronary Syndrome Funded by a Research Grant from Johnson and Johnson and Bayer to Brigham & Women’s Hospital. Dr. Gibson has received honoraria & consulting fees from J&J and Bayer. BACKGROUND: Thrombin In ACS • There is excess thrombin generation that persists for 6 months following an index ACS event.1 • Thrombin is the most potent stimulant of platelet aggregation.2 • Reduction of thrombin generation by warfarin reduces recurrent MI by 44% in a meta-analysis of 10 ACS trials.3 • Rivaroxaban is a direct factor Xa inhibitor which blocks initiation of the final common pathway leading to thrombin generation. • Based upon safety and efficacy in Phase II, 5.0 mg bid and 2.5 mg bid doses of Rivaroxaban were chosen for Phase III evaluation in ATLAS TIMI 51.4 1. Merlini PA et al. Circulation. 1994;90:61-68. 2. Coughlin S. Thrombin signaling and protease-activated receptors. Nature 2000;407(6801):258-64. 3. Rothberg MB et al Ann Intern Med. 2005 Aug 16;143(4):241-50. 4. Lancet. 2009;374(9683):29-38. TRIAL ORGANIZATION Trial Leadership: TIMI Study Group Chairman: Eugene Braunwald, Principal Investigator: C. Michael Gibson Investigator: Jessica Mega, Statisticians: Sabina Murphy, Charles Contant Executive Committee Jean-Pierre Bassand, Deepak Bhatt, Christoph Bode, Keith Fox, Marc Cohen, Shinya Goto, David Schneider, Freek Verheugt Sponsors: Johnson & Johnson and Bayer Health Care J&J: Paul Burton, Peter DiBattiste, Alexei N. Plotnikov, Linda DeCaprio, Xiang Sun Bayer: Nancy Cook Bruns, Scott Berkowitz, Frank Misselwitz Data Safety Monitoring Board Douglas Weaver (Chair) , Christian Hamm, Judith S. Hochman, Jeffrey Anderson, Hiroyuki Daida, Statistician: Allan Skene Recent ACS: STEMI, NSTEMI, UA No increased bleeding risk, No warfarin, No ICH, No prior stroke if on ASA + Thienopyridine Stabilized 1-7 Days Post-Index Event Stratified by Thienopyridine use at MD Discretion Placebo N=5,176 ASA + Thieno, n=4,821 ASA, n=355 + ASA 75 to 100 mg/day RIVAROXABAN RIVAROXABAN 2.5 mg BID 5.0 mg BID n=5,174 N=5,176 ASA + Thieno, n=4,825 ASA, n=349 ASA + Thieno, n=4,827 ASA, n=349 PRIMARY ENDPOINT: EFFICACY: CV Death, MI, Stroke* (Ischemic + Hemg.) SAFETY: TIMI major bleeding not associated with CABG Event driven trial of 1,002 events in 15,342 patients** * Stroke includes ischemic stroke, hemorrhagic stroke, and uncertain stroke ** 184 subjects were excluded from the efficacy analyses prior to unblinding NATIONAL LEAD INVESTIGATORS RUSSIA (1756) M. Ruda INDIA (1469) V. Chopra POLAND (1062) M. Tendera CHINA (901) R. Gao BULGARIA (792) N. Gotcheva UNITED STATES (684) C.M. Gibson UKRAINE (629) A. Parkhomenko BRAZIL (529) J. Nicolau ARGENTINA (404) M. Amuchastegui JAPAN (400) S. Goto NETHERLANDS (377) T. Oude Ophuis M. van Hessen ISRAEL (353) S. Meisel GERMANY (332) E. Giannitsis ROMANIA (304) D. Vinereanu COLOMBIA (269) R. Botero MEXICO (254) G. Llamas AUSTRALIA (510) P. Aylward CZECH REPUBLIC (485) P. Widimsky HUNGARY (412) R. Kiss UNITED KINGDOM (254) I. Squire ITALY (235) D. Ardissino SPAIN (230) A. Betriu 44 Countries CHILE (213) R. Corbalan FRANCE (213) G. Montalescot CANADA (190) M. Le May P. Theroux SLOVAKIA (178) T. Duris LITHUANIA (177) B. Petrauskiene TUNISIA (177) H. Haouala BELGIUM (173) F. Van de Werf EGYPT (159) A. Mowafy KOREA, REPUBLIC OF (150) K. Seung SWEDEN (144) M. Dellborg THAILAND (140) P. Sritara 766 Sites TURKEY (119) Z. Yigit SERBIA (117) Z. Vasiljevic PORTUGAL (115) J. Morais LATVIA (100) A. Erglis DENMARK (99) S. Eggert Jensen NEW ZEALAND (98) H. White MALAYSIA (97) K. Hian Sim GREECE (69) CROATIA (62) M. Bergovec MOROCCO (57) PHILIPPINES (38) BASELINE CHARACTERISTICS Placebo Rivaroxaban Rivaroxaban 2.5 mg BID 5.0 mg BID Age, mean (SD) 61.5 (± 9.4) 61.8 (± 9.2) 61.9 (± 9.0) Sex, male n (%) 75.0% 74.9% 74.2% Prior MI, n (%) 27.3% 26.3% 27.1% Diabetes, n (%) 31.8% 32.3% 31.8% STEMI, n (%) 50.9% 50.3% 49.9% NSTEMI, n (%) 25.6% 25.5% 25.8% UA, n (%) 23.6% 24.2% 24.3% PCI at Index Hosp, n (%) 59.9% 60.2% 60.0% STATISTICAL ANALYSIS The primary efficacy endpoint of CV death, MI and stroke* (ischemic + hemorrhagic) was evaluated sequentially**: • Pre-Specified Analysis: Rivaroxaban 2.5 + 5.0 mg BID doses pooled together across both thienopyridine strata (all Rivaroxaban vs all Placebo) If p < 0.0499982 interim adjusted • Rivaroxaban 2.5 mg BID and 5.0 mg BID doses separately across both thienopyridine strata (p<0.05) • The primary method of analysis was a log rank test stratified by thienopyridine use in the mITT# population with confirmation in an ITT## analysis * Stroke includes ischemic stroke, hemorrhagic stroke, and uncertain stroke ** Same testing procedure was conducted independently for ASA+Thienopyridine # mITT, all randomized subjects and end point events, which are observed from randomization up to the earliest date of the completion of the treatment period (i.e, global treatment end date), or 30 days after early discontinuation of the study drug or 30 days following randomization for those subjects who were randomly assigned to treatment but not treated ## ITT, all randomized subjects and end point events, which are observed from randomization up to the global treatment end date PRIMARY EFFICACY ENDPOINT: CV Death / MI / Stroke* (Ischemic + Hemg.) 2 Yr KM Estimate Estimated Cumulative Rate (%) Placebo No. at Risk Placebo Rivaroxaban 10.7% 8.9% HR 0.84 (0.74-0.96) ARR 1.7% Rivaroxaban (both doses) mITT p = 0.008 ITT p = 0.002 NNT = 59 Months After Randomization 5113 4307 3470 2664 1831 1079 421 10229 8502 6753 5137 3554 2084 831 *: First occurrence of cardiovascular death, MI, stroke (ischemic, hemorrhagic, and uncertain) as adjudicated by the CEC across thienopyridine use strata Two year Kaplan-Meier estimates, HR and 95% confidence interval estimates from Cox model stratified by thienopyridine use are provided per mITT approach; Stratified log-rank p-values are provided for both mITT and ITT approaches; ARR=Absolute Relative Reduction; NNT=Number needed to treat; Rivaroxaban=Pooled Rivaroxaban 2.5 mg BID and 5 mg BID. STENT THROMBOSIS* ARC Definite, Probable, Possible Estimated Cumulative incidence (%) 2 Yr KM Estimate Placebo 2.9% 2.3% HR 0.69 Rivaroxaban (both doses) ARC Definite/probable: HR=0.65, mITT p=0.017, ITT p=0.012 (0.51- 0.93) mITT p = 0.016 ITT p = 0.008 Months After Randomization * End point events are as adjudicated by the CEC across thienopyridine use strata Two year Kaplan-Meier estimates, HR and 95% confidence interval estimates from Cox model stratified by thienopyridine use are provided per mITT approach; Stratified log-rank p-values are provided for both mITT and ITT approaches; Rivaroxaban=Pooled Rivaroxaban 2.5 mg BID and 5 mg BID. PRIMARY EFFICACY ENDPOINT: 5.0 mg BID CV Death / MI / Stroke* (Ischemic + Hemg.) Estimated Cumulative incidence (%) Placebo 10.7% 10 8.8% HR 0.85 mITT p=0.028 5 Rivaroxaban 5 mg BID ITT P=0.010 0 0 12 24 Months Rivaroxaban at 5 mg PO BID was associated with a numerical but not statistically significant reduction in mortality. * First occurrence of cardiovascular death, MI, stroke (ischemic, hemorrhagic, and uncertain) as adjudicated by the CEC Two year Kaplan-Meier estimates, HR and 95% confidence interval estimates from Cox model stratified by thienopyridine use are provided per mITT approach; Stratified log-rank p-values are provided for both mITT and ITT approaches. PRIMARY EFFICACY ENDPOINT*: 2.5 mg PO BID CV Death / MI / Stroke* All Cause Death Cardiovascular Death 5% 5% Estimated Cumulative incidence (%) 12% HR 0.84 HR 0.66 Placebo 10.7% mITT p=0.020 4.1% mITT p=0.002 9.1% ITT p=0.007 HR 0.68 Placebo Placebo mITT p=0.002 4.5% ITT p=0.004 ITT p=0.005 2.9% 2.7% 0 Rivaroxaban 2.5 mg BID Rivaroxaban 2.5 mg BID Rivaroxaban 2.5 mg BID NNT = 63 NNT = 71 NNT = 63 12 Months 24 0 12 Months 24 0 12 Months 24 * First occurrence of cardiovascular death, MI, stroke (ischemic, hemorrhagic, and uncertain) as adjudicated by the CEC across thienopyridine use strata Two year Kaplan-Meier estimates, HR and 95% confidence interval estimates from Cox model stratified by thienopyridine use are provided per mITT approach; Stratified log-rank p-values are provided for both mITT and ITT approaches; NNT=Number needed to treat. PRIMARY EFFICACY ENDPOINTS: 2.5 mg PO BID In Patients Treated with ASA + Thienopyridine CV Death / MI / Stroke* Estimated Cumulative incidence (%) 12% HR 0.85 All Cause Death Cardiovascular Death 5% 5% HR 0.62 Placebo mITT p=0.039 mITT p<0.001 10.4% HR 0.64 Placebo 4.2% Placebo mITT p<0.001 4.5% 9.0% ITT p=0.011 ITT p<0.001 ITT p<0.001 2.7% 2.5% 0 Rivaroxaban 2.5 mg BID Rivaroxaban 2.5 mg BID Rivaroxaban 2.5 mg BID NNT = 71 NNT = 59 NNT = 56 12 Months 24 0 12 Months 24 0 12 Months *: First occurrence of cardiovascular death, MI, stroke (ischemic, hemorrhagic, and uncertain) as adjudicated by the CEC Two year Kaplan-Meier estimates, HR and 95% confidence interval estimates from Cox model stratified by thienopyridine use are provided per mITT approach; Stratified log-rank p-values are provided for both mITT and ITT approaches; NNT=Number needed to treat. 24 PRIMARY EFFICACY SUBGROUP RESULTS (mITT) HR (95% CI) Pinteraction Overall 0.84 (0.74 0.96) ASA ASA + thienopyridine 0.69 (0.45 -1.05) 0.86 (0.75 -0.98) 0.34 <65 Years 65 Years 0.83 (0.70 - 0.99) 0.94 STEMI NSTEMI UA 0.85 (0.70 - 1.03) 0.85 (0.68 - 1.06) 0.82 (0.62 - 1.07) 0.96 Male Female 0.87 (0.75 - 1.01) 0.40 Weight <60 kg Weight 60 to <90 kg Weight 90 kg 0.83 (0.56 - 1.25) Prior MI No Prior MI 0.83 (0.68 - 1.01) Diabetes Mellitus No Diabetes Mellitus 0.96 (0.77 - 1.20) Creatinine Cl <50mL /min Creatinine Cl >50 mL /min 0.88 (0.62 - 1.26) North America South America Western Europe Eastern Europe Asia Other 0.57 (0.33 - 0.97) 0.84 (0.70 - 1.01) 0.77 (0.60 - 0.99) 0.98 0.85 (0.72 - 0.99) 0.83 (0.64 - 1.08) 0.80 0.85 (0.72 - 1.01) 0.14 0.78 (0.67 - 0.92) 0.82 0.84 (0.73 - 0.96) 0.89 (0.59 - 1.34) 0.90 (0.59 - 1.37) 0.83 (0.69 - 1.00) 0.86 (0.63 - 1.17) 0.92 (0.60 - 1.39) 0.5 0.8 Rivaroxaban Better 1.0 1.25 2.0 Placebo Better 0.80 SAFETY ENDPOINTS Treatment-Emergent Non CABG TIMI Major Bleeding* Analysis 2 Yr KM Estimate Placebo 2.5 mg Rivaroxaban 5.0 mg Rivaroxaban 0.6% 1.8% 2.4% HR 3.46 HR 4.47 p<0.001 p<0.001 Post-Treatment Ischemic Events# 1-10 Days After Last Dose 1.8% 1.4% 2.2% p=NS p=NS Liver Function Test (ALT > 3xULN) ## Treatment-Emergent 1.6% 1.3% 1.4% p=NS p=NS There was no excess of either combined ALT > 3x ULN and Total Bilirubin > 2x ULN cases among patients treated with Rivaroxaban, or SAEs. *: First occurrence of Non-CABG TIMI major bleeding events occurred between first dose to 2 days post last dose as adjudicated by the CEC across thienopyridine use strata; Two year Kaplan-Meier estimates, HR and 95% confidence interval estimates from Cox model stratified by thienopyridine are provided; Stratified log-rank p-values are provided; #: Raw percentage for CV death/MI/stroke (ischemic, hemorrhagic, uncertain) ; ##: Raw percentage of subjects with abnormal value measured between first dose to 2 days post last dose among subjects with normal baseline measurement. TREATMENT-EMERGENT FATAL BLEEDS AND ICH 1.2 1 0.8 0.6 p=NS for Riva vs Placebo 0.7 p=0.044 for 2.5 mg vs 5.0 mg 0.4 0.4 0.2 0 p=0.009 Riva Vs Placebo Placebo 2.5 mg Rivaroxaban 5.0 mg Rivaroxaban 0.2 0.4 p=NS for Riva vs Placebo 0.2 0.2 0.1* 0.1 0.1 n=9 n=6 n=15 n=5 n=14 n=18 n=4 n=5 n=8 Fatal ICH Fatal ICH *Among patients treated with aspirin + thienopyridine, there was an increase in fatal bleeding among patients treated with 5.0 mg of Rivaroxaban (15/5110) vs 2.5 mg of Rivaroxaban (5/5115) (p=0.02) SUMMARY- SAFETY • There was a dose dependent increase in bleeding associated with rivaroxaban (2.5 mg ↓ 5.0 mg). • Although ICH was increased with rivaroxaban, there was no excess risk of fatal ICH or fatal bleeding associated with rivaroxaban compared to placebo. • No evidence of drug induced liver injury or rebound (post-treatment) ischemic events SUMMARY-EFFICACY • The primary efficacy endpoint of CV death, MI and stroke was reduced when added to standard therapy for both rivaroxaban doses combined, and for the 2.5 and 5.0 mg BID doses separately • CV and all cause death were reduced for both rivaroxaban doses combined, and for the 2.5 mg BID dose in both mITT and ITT analyses SUMMARY-EFFICACY (cont.) • When 2.5 mg PO BID of rivaroxaban was added to ASA + thienopyridine, cardiovascular death was reduced by 38% and all cause death by 36% • One death prevented if 56 patients treated for two years with 2.5 mg BID of Rivaroxaban