So, what is Chemistry? What does the word “chemistry” make you
advertisement
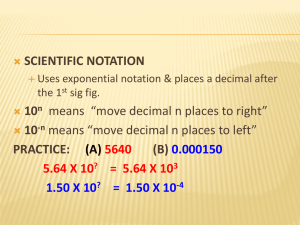
So, what is Chemistry? What does the word “chemistry” make you think? This? Or this? Or maybe this? And possibly this.. But, Chemistry really is… Baby stuff! And… • Cool cars! And… • Things you need And… • Things that you really need And.. • Things that some of us really, really need.. And… • Fun stuff… But, seriously.. • How would we survive without it? This is a part of chemistry So is this.. And this.. Chapter 1 Notes • Math requirements for general chemistry: 1. Significant figures and usage 2. Scientific notation and usage 3. Basic algebra 4. Dimensional analysis 5. Graphing and interpretation LO’s for SigFigs and SciNot • Explain the meaning of and identify significant figures in experimental data. • Correctly perform calculations using significant figures • Convert any number to or from scientific notation • Correctly perform calculations using scientific notation with a calculator. Significant Figures Significant Figures are affectionately referred to as SigFigs by chemists. SigFigs are used as a method to convey how precisely a measured quantity is known. SigFigs can tell you how carefully a quantity was measured. All experimental data has some uncertainty associated with it, usually due to the limitations of the equipment used to obtain the data. The mass of a beaker was measured on 3 different balances. Which value is correct? Which digit is uncertain? 2.3 g 2.31 g 2.3145 g What would the 3 balances read if you had a beaker with a mass of exactly 2500 g? Rules for Finding SigFigs 1. All non-zero digits are always Sig. 2. All captive zeros are always Sig. 3. Leading zeros (to the left) are Never Sig 4. Trailing zeros (to the right) are only Sig when the number has a decimal point. 5. Counting numbers have infinite SigFigs. Practice- How many SigFigs? 4509 -____ 1.007 - ____ 4510 -____ 10.0 - ____ 12,000 -_____ 0.00501 - ____ 120,020 -_____ 0.02100 - _____ Doing math with SigFigs Multiplication and division: - Count the number of SigFigs in each factor. Your answer will have the smallest number of SigFigs in the factors. - Examples: 2.000 x 5.00 = 10.0 - 3.14 x 2.2 = 6.908 = 6.9 - 1500 / 6.24 = 240.3846 = 240 - 100000 / 75.202 = 1329.752=1000 Addition and subtraction: Add or subtract the numbers as normal. To determine the number of sigfigs, start from the left end of your answer and check, place by place (hundreds, tens, ones, tenths) if there are sigfigs in that place for all factors. When 1 factor runs out of sigfigs, all sigfigs to the right in your answer are dropped. (Ignore empty spaces to the left of the decimal point). Examples: 2010 41.75 +2.1 2053.85 = 2050 1.204 0.0072 +5.25 6.4612 = 6.46 200,000 -10.0 199,990.0=200,000 Scientific notation Serves two purposes: 1. To simplify writing very large (6.02 x 1023) and very small (1.6 x 10-26) numbers. 2. To clarify confusion about the number of SigFigs in a value. 2100 versus 2100. 1,000,000 vs 1,000,000. For numbers larger than 1: Re-write the number dropping all zeros that are not sigfigs. Put in a decimal place so that there is one and only one digit to the left of the decimal place. Write “x10” after the number. • Ex: 107,000 = 1.07 x 10 Count the number of places that the decimal place must be moved to get from the original number to the re-written number. Write the number of places moved as the exponent for the 10. • Ex: 107,000 moves 5 places so we write 1.07 x 105 For numbers less than 1: The rules are exactly the same except that the exponent is written as a negative number. • Ex: 0.00107 moves three places so we write1.07 x 10-3 Practice: 12,000 = 6.1 x 104 = 1,060,000 = 2.07 x 107= 0.0021 = 4.04 x 10-3= 0.0003050 = 2.90 x 10-4= • Do this calculation with your calculator!!! 6.25x108 / 1.70x10-4 SciNot Using Calculators Do not use the “carrot key” Ʌ Enter the number (ex: for 6.02 x1023) 6.02 Press 2nd EE (on the TI30)–do not enter x 10 Enter the exponent 23 Your calculator should read 6.02E23 The “E” means “x10 to the” Practice 6.02x1023 / 4.5x1012 = 1.75x10-5 x 2.17x108 = (4.05x106) (3.1x10-7) / 8.82x1015 = Basic Algebra In chemistry, you will often have problems where you must solve an algebraic equation for an unknown. You must be familiar with how to do this. Brief review: M D= V Solve for M Solve for V To solve for an unknown, you must get the unknown by itself, on top, on one side of the equal sign. Move either the unknown or the other variables until solved. When moving variables (multiplication and division) a variable on top (numerator) on one side of the equals goes to the bottom (denominator) on the other side. A variable on the bottom goes to the top when moved to the other side. Practice: ax = b y t 2.5z = 15 PV=nRT Finally, Chemistry!!!! • • • • • • Chemistry Learning objectives Chapter 1-B Differentiate between and discuss characteristics of 3 common states of matter Model structure of 3 states of matter Identify products and reactants in a chemical reaction Differentiate between and give examples of chemical and physical changes Differentiate between and give examples of endothermic and exothermic reactions Discuss energy changes in products and reactants for exo and endothermic reactions • Chemistry is defined as the study of matter and the changes matter undergoes. • Matter – Anything that has mass and volume (takes up space) • Essentially, chemistry is the study of everything in the universe and what happens to it Physical States of matter Solid Particles closely packed Liquid Gas Closely packed Very loose Strong attraction Medium between particles attraction Weak to no attraction Fixed shape No fixed shape No fixed shape Fixed volume Fixed volume No fixed volume Physical change – A change where the chemical identity of the material does not change. Ex: Chemical change – A change where new substances are formed. The chemical identity does change. Ex: Reversible and not reversible are not good indicators of physical and chemical changes Chemical Reactions In any chemical reaction, atoms are rearranged to form different substances! No new atoms are formed – no atoms are destroyed. All reactions are written as: Reactants → Products Reactants – left side of arrow – what you are starting with. Products – Substances that are produced in the reaction. Right side of the arrow. → - The action!!! Can be read as “yields” “reacts to form” “produces” “gives” “decomposes to” Examples: Exothermic reactions: Energy is released as a products of the reaction. Energy stored in the reactants is released to the surroundings (beaker gets HOT). Example: Endothermic reactions: Energy is absorbed from the surroundings and added to the reactants. (beaker gets cold) Example: Graphs: Signs of a chemical reaction 1. 2. 3. 4. 5. Giving off a gas. Precipitation – Formation of a solid. Change in heat. Absorbed or given off. Production of light. Change in color. Units and the SI system In science, values or numbers do not occur by themselves. A number must have a unit with it to give the number meaning. Think of 3!!!! Seven SI base units • • • • • The ampere (A) - unit of measurement of electric current The kilogram (kg) - unit of measurement of mass The metre (m) - unit of measurement of length The second (s) - unit of measurement of time The kelvin (K) - unit of measurement of thermodynamic temperature • The mole (mol) - unit of measurement of amount of substance • The candela (cd) - unit of measurement of luminous intensity Units that you should be familiar with Milliliter (mL)– volume – about _____ drops Liter (L)– volume – a little more than __ quart Gram (g)– mass – a penny has a mass of __ g Kilogram (kg)– a little more than ___ pounds Meter (m)– distance - a little more than a yard Centimeter (cm)– distance - a little less than ____inch Dimensional Analysis A method of calculation that uses the UNITS of the values and conversion factors to set up the calculation. A conversion factor is any mathematical relationship between two units. Used to convert from one unit to another. Examples: How to do Dimensional analysis: 1. In the problem, find the UNITS that you are looking for and the UNITS that you are given. 2. Find an appropriate conversion factor (or series of factors) that will allow you to relate given units to looked for units. 3. Draw a grid and place the given unit in the upper left. Enter conversion factor into the grid so that units cancel (top and bottom) until only the looked for unit is left. 4. Put in the numbers in appropriate places and run them thru your calculator for the answer. Example 1: An elephant weighs 12 tons. How many pounds is this? Looked for unit – Given unit – Conversion factor – Example 2: The fence in a baseball field in 330 ft from home plate. How far is this in yards? Looked for units – Given units – Conversion factor - Example 3: A month is 30 days long. How long is this in hours? In minutes? In seconds? Looked for units – Given units – Conversion factor - How old are you in seconds? SI unit conversions – The prefix tells you the conversion factor. All prefixes are used the same way. Ex: Milli means 1/1000 (10-3). This means that: There are 1000 milligrams in 1 gram Or 1 milligram is 1x10-3 grams 1000 mg 1g 1g 1 mg 1000 mg 10-3 g Practice Properties of matter Properties are characteristics or descriptions of matter. 1. Chemical properties – Can only be observed when a substance changes. Tells how a substance reacts with others. 2. Physical properties – Can be measured or observed without changing the substance. Chemical property examples: Physical property examples: • Chemical change – Produces new substances. The chemical identity of substances change. • Examples: • Physical Change – Only affects the physical properties. No new substances formed. • Examples: Density-A physical property • Density is defined as the amount of mass per unit of volume. • Can be thought of as the amount of matter packed into a given volume. • Most common units are g/mL • May often see g/L or g/cm3 D= M V Notice that density relates the mass of a material to the volume. It can be used as a conversion factor in dimensional analysis to convert between mass and volume. Practice 1: 50.0 mL of gold has a mass of 965g. What is the density of gold? Practice 2: Helium has a density of 0.179 g/L. A balloon that holds 2.5 L of He would have what mass of He? Practice 3: A chunk of gold that weighs 2 lbs would take up how much space? (1 lb = 454 g) The Nature of Matter Atom – The basic building block of matter. Think Legos!!!! There are 92 different kinds of atoms that occur naturally on earth. (We will have much, much more to say about this!!!!) Element – A substance that is composed of only 1 type of atom. Cannot be broken down into a simpler type of matter by any chemical means. • Molecules – Two or more atoms that are bonded together. (Snapping Legos together). If the same type of atoms are bonded then the molecule makes up an element. • Compounds – Two or more different types of atoms bonded together. Can be broken down into simpler substances by chemical change. Properties are different than those of component elements. Always a fixed ratio of elements (must have a formula). • Mixtures – Two or more compounds and/or elements physically mixed but not chemically bonded. Can be separated by physical means. Keeps the properties of the component substances. Homogeneous mixtures – Same throughout Heterogeneous mixtures – Different components are apparent. Matter Pure substance Element Compound Mixture Homogeneous Heterogeneous • Some elements are diatomic – They occur in nature as molecules composed of 2 atoms. You should remember these 7 Diatomics!!! Bromine Oxygen Fluorine Iodine The BOFINCH elements Nitrogen Chlorine Hydrogen • Allotrope – Different molecular or crystalline form of the same element. Examples: