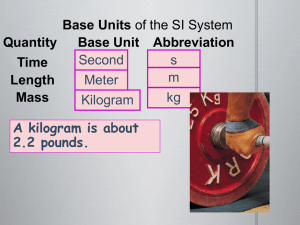
THE SI system and units of measurement
advertisement

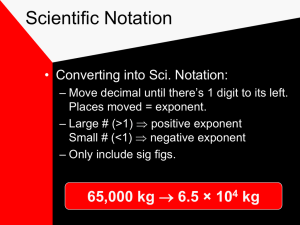
SCIENTIFIC NOTATION Uses exponential notation & places a decimal after the 1st sig fig. 10n means “move decimal n places to right” 10-n means “move decimal n places to left” PRACTICE: (A) 5640 (B) 0.000150 5.64 X 10? = 5.64 X 103 1.50 X 10? = 1.50 X 10-4 COUNTING SIGFIGS IN SCIENTIFIC NOTATION EXPONENTIAL TERM – NO SIG FIGS (IT ONLY MOVES THE DECIMAL PLACE) NUMBER-CONTAINS ONLY SIF FIGS EX. 7.809 X 104 4 SIG FIGS 3.40 X 10-6 3 SIG FIGS What is the correct mass to report? Measured Mass is _______________ SIGNIFICANT FIGURES Rules for recognizing SigFigs: When a number has a decimal: Read Left to Right until you Reach the first nonzero number Then you begin counting SigFigs Until you run out of digits (including any zeros along the way) Example: 0.003210 g = _______ SigFigs? SIGNIFICANT FIGURES Practice: 82.700 K 0.000365 J 3.034 g 3.220 x 105 m ____sig figs ____sig figs ____sig figs ____sig figs SIGNIFICANT FIGURES Rules for recognizing SigFigs: When a number has no decimal: Read Right to Left until you Reach the first nonzero number Then you begin counting SigFigs Until you run out of digits (including any zeros along the way) Example: 450300 m = _______ SigFigs? SIGNIFICANT FIGURES Practice: 421 m 800,890 J 39,059,000 g 523,000 L ____ sig figs ____sig figs ____sig figs ____sig figs SIGNIFICANT FIGURES Counted Quantites and Constants have absolute values & we don’t count SigFigs Either you counted the number of items right, or you didn’t! Example: 14 bananas SIGNIFICANT FIGURES 1. 2. 3. 4. 5. How many significant figures are in each of the following numbers? 5.40 ng 1.2 x 103 mt 210 N 0.00120 mL 801.5 K ____ ____ ____ ____ ____ 6. 7. 8. 9. 10. 0.0102 Amp 1,000 BTU 9.010 x 10-6 dm 101.0100 D 2,370.0 Cal ____ ____ ____ ____ ____ SIGNIFICANT FIGURES Why are significant figures important when taking data in the laboratory? When you measure something, you never get 100% accuracy on its measurement, so you need a way to show just how accurate it is. SIGNIFICANT FIGURES Why are significant figures NOT important when solving problems in your math class? In math, we assume that all numbers are either 100% accurate, or that they are constants, and thus have no uncertainty or error involved in their creation. SIGNFICANT FIGURES AND MATH We will be required to do math with our SigFigs Two rule sets cover this: Addition/Subtraction And Multiplication/Division ADDITION AND SUBTRACTION SIG FIG RULES line up the decimals 2. draw a line to the right of the number with the least precision (shortest column) 3. answer can’t go past the line 4. use the 1st digit past the line to determine where to round off Example: 32.00 m + 48.1 m + 182.213 m = ???? 1. ADDITION AND SUBTRACTION SIG FIG RULES line up the decimals 2. draw a line to the right of the number with the least precision (shortest column) 3. answer can’t go past the line 4. use the 1st digit past the line to determine where to round off Example: 32.00 m + 48.1 m + 182.213 m = ???? 1. 32.00 m 48.1 m +____________ 182.213 m 262.313 m Must round to: 262.3 m ADDITION AND SUBTRACTION SIG FIG RULES 1. 210.6 mm + 14.57 mm = ____________ 2. 74.000 cm + 8.6 cm =____________ 3. 0.0787 m + 0.85 m =____________ 4. 84000 cm + 1110 cm =____________ 5. 92008 g + 32100 g =____________ 6. 8.000 mm + 0.0304 m =____________ 7. 84.34 g - 5.2 g =____________ 8. 9.81 cm - 3.151 cm =____________ 9. 0.0900 n - 0.0094 n =____________ MULTIPLYING AND DIVIDING SIG FIG RULES Answer can have no more SigFigs than the factor with the fewest number of SigFigs. This means that constants and quantities do not affect sigfigs in the answer! Example: 127.3 x 42 = ??? MULTIPLYING AND DIVIDING SIG FIG RULES 1. 500 kg X 32 kg =____________ 2. 680.0 n X 100. n =____________ 3. 8560.0 g X 1000 g =____________ 4. 4560 m X 0.100 m =____________ 5. 85 kg X 0.001 kg =____________ 6. 9200 L ÷ 873 L =____________ 7. 0.85 2 kg2 ÷ 62 kg =____________ 8. 985 g2 ÷ 500. g =____________ 9. 10000 n2 ÷ 0.10 n =____________ 10. 0.0006 g2 ÷ 25 g=____________