Chapter 4 - Relative Atomic Mass and the Mole
advertisement

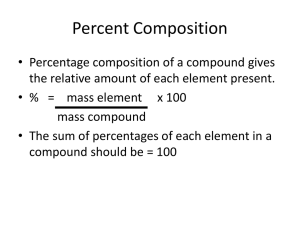
Chapter 4: Relative Atomic Mass and the Mole Week 4, Lesson 1 Masses of Particles Relative Isotopic Mass • Chemists as early as John Dalton, two centuries ago, used experimental data to determine the weight of different atoms relative to one another. • Dalton estimated relative atomic weights based on a value of one unit for the hydrogen atom. • In 1961, it was decided that the most common isotope of 12C would be used as the reference standard. • On this scale, the 12C isotope is given a relative mass of exactly 12 units. Relative Isotopic Mass cont… “The relative isotopic mass (Ir) of an isotope is the mass of an atom of the that isotope relative to the mass of an atom of 12C taken as 12 units exactly.” Isotopic Masses example… • Chlorine has two isotopes. • These have different masses as they have different amounts of neutrons. • Using the 12C isotope as a standard, the relative isotopic masses of these two isotopes are 34.969 (35Cl) and 36.966 (37Cl). • Naturally occurring chlorine is made of 75.80% of the lighter isotope and 24.20% of the heavier isotope. Isotopic Composition of Some Common Elements ELEMENT ISOTOPES RELATIVE ISOTOPIC MASS ABUNDANCE (%) Hydrogen 1H 1.008 99.986 2H 2.014 0.014 3H 3.016 0.001 12C 12 exactly 98.888 13C 13.003 1.112 14C 14.003 Approx 10-10 16O 15.995 99.76 17O 16.999 0.04 18O 17.999 0.20 107Ag 106.9 51.8 108Ag 108.9 48.2 Carbon Oxygen Silver Mass Spectrometer • Relative isotopic masses of elements can be obtained using an instrument called a mass spectrometer. • This separates the individual isotopes in a sample of the element and determines the mass of each isotope. • The information is presented graphically and is known as a mass spectrum. Mass Spectrometer cont… • In a mass spectrum showing the isotopes of an element: – The number of peaks indicates the number of isotopes – The position of each peak on the horizontal axis indicates the relative isotopic mass – The relative heights of the peaks correspond to the relative abundance of the isotopes Relative Atomic Mass • A naturally occurring sample of an element contains the same isotopes in the same proportions regardless of its source. • It is sufficient to imagine that the hypothetical average atoms of the element are being used. • This average is known as the relative atomic mass. • Its symbol is Ar. Relative Atomic Mass cont… • The relative atomic mass of an element is actually a weighted average of the relative isotopic masses because it takes into account the relative amounts or abundances of each isotope in natural samples of the element. Average Relative Mass example… 1. Imagine taking 100 atoms from a sample of chlorine of chlorine – there will be 75.80 atoms of 35Cl and 24.20 of 37Cl. Find the relative atomic mass… Equation to use: ((relative isotopic mass1 x % abundance1) + (relative isotopic mass2 x % abundance2)) /100 OR Ar = ∑(relative isotopic mass x %abundance) / 100 Average Atomic Mass cont… • Imagine taking 100 atoms from a sample of chlorine of chlorine – there will be 75.80 atoms of 35Cl and 24.20 of 37Cl. Find the relative atomic mass… Ar = 34.969 75.80 36.966 24.20 100 Ar = 2650.65 894.58 100 Ar = 35.452 Now you try… • Find the relative atomic mass of the following… 1.Hydrogen 2.Carbon 3.Oxygen 4.Silver Equations with mass spectrums… • A simplified mass spectrum of magnesium is shown on page 58 (of textbook). From the mass spectrum calculate the percentage abundances of each of its three isotopes. - The heights of the peaks for the graph are: - 24Mg = 7.9 cm 25Mg = 1.0 cm 26Mg = 1.1 cm Equations with mass spectrums cont… • So, the percentage of the 24Mg isotope = 7.9 100% = 79% 7.91.01.1 • The percentage of 25Mg isotope = = 10% 1.0 100 7.9 1.0 1.1 • The percentage of 26Mg isotope 1.1 = 11% = 100 7.9 1.0 1.1 Percentage Abundance example… • Copper has two isotopes. 63Cu has a relative isotopic mass of 62.95 and 65Cu has a relative isotopic mass of 64.95. The relative atomic mass of copper is 63.54. Calculate the percentage abundance of the two isotopes. 1.Let x be the percentage abundance of 63Cu 2.So, 100-x is the percentage abundance of 65Cu Percentage Abundance example… 3. Ar(Cu) = ∑(relative isotopic mass x %abundance) 100 So 63.54 = 62.95x 64.95(100 x) 100 6354 = 62.95x + 6495 – 64.95x 6354 = 6495 – 2x 2x = 6495 – 6354 2x = 141 x = 70.5 Relative Molecular Mass • The relative molecular mass (Mr) of a compound is the mass of one molecule of that substance relative to the mass of a 12C atom. • The Mr is calculated by taking the sum of the relative atomic masses of the elements in the molecular formula. Relative Molecular Mass cont… Calculate the Mr of CO2: Mr(CO2) = Ar(C) + 2 x Ar (O) = 12.0 + 2 x 16.0 = 44.0 For non-molecular compounds the term relative formula mass is used. This is solved exactly the same way. Now you try… Calculate the Mr of the following: 1.NaCl 2.H2O 3.H2SO4 Week 4, Lesson 2 The Mole • A mole is defined as the amount of substance that contains the same number of specified particles as there are atoms in 12g of carbon12. The mole has been defined in a convenient way: – 1 atom of 12C has a relative atomic mass of 12 exactly – 1 mol of atoms of 12C has a mass of 12g exactly. The Mole cont… • The number of particles in 1 mole is given by the symbol NA. • Because atoms and molecules are so small, NA, needs to be a very large number if 1 mole of a substance is to be an amount that is convenient to work with. • It is for this reason that chemists have selected 12g of Carbon-12 atoms to define the mole. Symbols and Units • The symbol for the amount of substance is n. • The unit of measurement for the amount of substance is mol. • NA, also known as Avagadro’s Constant, = 6.02 x 1023 • Therefore 1 mole of particles equals 6.02 x 1023. • If given the amount of substance, we can calculate the number of particles. N = n x NA • N = number of particles • n = amount of substance • NA = Avagadro’s Constant • Find the number of O2 molecules in 2.5 mol of oxygen. – N = n x NA – N = 2.5 x 6.02 x 1023 – N = 15.05 x 1023 – N = 1.5 x 1024 Rearranging the Equation • You can also use this equation to find the amount of substance, in mol, by rearranging the equation. N n NA Molar Mass • The mass of 1 mole of a particular element or compound is known as the molar mass. • Because the particles of different elements or compounds have different mass, the masses of 1 mol samples of different particles will be different. • For example, one dozen eggs. Molar Mass cont… • In general – The molar mass of an element is the relative atomic mass of the relative atomic mass expressed in grams. – The molar mass of a compound is the relative molecular or relative formula mass of the compound expressed in grams. • The symbol for Molar Mass is M and its unit of measurment is gmol-1. Finding the Molar Mass • Calculate the molar mass of table sugar, sucrose (C12H22O11) 1. What are the mass numbers of the individual elements? C = 12.0 H=1 O = 16 2. Find the molar mass of each element. C = 12 x 12 H = 22 x 1 O = 11 x 16 3. Add them together. Mr = (12x12) + (22x1) + (11x16) Mr = 342g mol-1 n = m / M or m = n x Mr • • • • m = mass n = amount of substance M = Mr = molar mass This means that is we have the mass of substance we can find the amount in mol by substituting the mass in for m and working out the molar mass. • Or, we can find the mass if the amount of substance is known. Week 4, Lesson 3 Formulas of Compounds • Percentage Composition – The values for molar masses of elements in compounds can be used to calculate the percentage composition of a compound once if formula is known. – For example if a company is producing aluminum from alumina (Al2O3), the management would want to know the mass of aluminium that can be extracted from a given quantity of alumina. Percentage Composition example • Calculate the percentage of aluminium in alumina. 1. Find the molar mass of Al2O3. M(Al2O3) = (2 x 27) + (3 x 16) = 102gmol-1 2. Find the percentage of aluminium in alumina Since 1 mol of alumina contains 2 mol of Al atoms: Mass of aluminium in 1 mol (102g) of Al2O3 = 2x27 = 54g Percentage of Al in Al2O3 = mass of element in 1 mol of compound mass of 1 mole of compound 100 = 54/102 x 100 = 52.9% x Percentage Composition Formula % by mass of the element = mass of element in 1 mole of compound Mass of 1 mole of compound x100 Empirical Formulas • The empirical formula of a compound is the formula that gives the simplest whole number ratio, by number of moles, of each element in the compound. • Empirical formulas are determined experimentally usually by determining the mass of each element present in a given mass of compound. Empirical Formulas Process 1. Measure the mass (m) of each element in the compound. 2. Calculate the amount in mol (n) of each element in the compound. 3. Calculate the simplest whole number ratio of moles of each element in compound. 4. Empirical formula of compound. Empirical Formula Example 1 • A compound of carbon and oxygen is found to be 27.3% carbon and 72.7% oxygen by mass. Calculate the empirical formula. C : O 1. Mass 27.3 : 72.7 2. Moles 27.3 72.7 12 1. Find ratio 2.27 2.27 1 2.27 1. Ratio 1 : 2. Empirical Formula is CO2 4.54 16 4.54 2 2.27 2 Empirical Formula Example 2 • 9.0g of a compound of only carbon, hydrogen and oxygen is found to contain 4.8g of oxygen and 3.6g of carbon. Calculate the empirical formula. 1.Find the mass of hydrogen: 9.0 = 4.8 + 3.6 + m(H) 9.0 = 8.4 + m(H) M(H) = 0.6g Empirical Formula Example 2 cont… Carbon Hydrogen Oxygen Step 1: mass (g) 3.6 0.6 4.8 Step 2: mol (n) 3.6 12.0 = 0.3 0.6 1.0 = 0.6 4.8 16.0 = 0.3 0.3 0.3 0.6 0.3 =2 0.3 0.3 Step 3: Find ratio Step 4: Give simplest ration 1 =1 2 Therefore, the empirical formula is CH2O. 1 = 1 Molecular Formulas • While an empirical formula give the simplest whole number ratio of an element in a compound, a molecular formula gives the actual number of atoms in one molecule of the compound. • The empirical and molecular formula can be the same or different. • The molecular formula is always a whole number multiple of the empirical formula. • A molecular formula can be obtained from the empirical formula if the molar mass of a compound is known. Molecular Formula Example 1 • A sample of a hydrocarbon was found to contain 7.2g of carbon and 1.5g of hydrogen. The molar mass of this compound was determined to be 58g mol-1. What is the molecular formula of the compound? Example cont… • Determine the empirical formula. Carbon Hydrogen Step 1: m(g) 7.2 1.5 Step 2: n (mol) 7.2 12 = 0.6 1.5 1.0 = 1.5 Step 3: Divide by smallest number 0.6 0.6 = 1 1.5 0.6 = 2.5 Step 4: Give simplest whole number ratio 2 5 • So the empirical formula is C2H5 Example cont… • Determine the molecular formula. – Empirical Formula is C2H5 – Molar Mass (C2H5) unit = 29g mol-1 – But molar mass of compound = 58g mol-1 – Number of C2H5 units in one molecule = 58 29 =2 Molecular formula = C2H5 x 2 = C4H10