IISc solidification lecture 4
advertisement
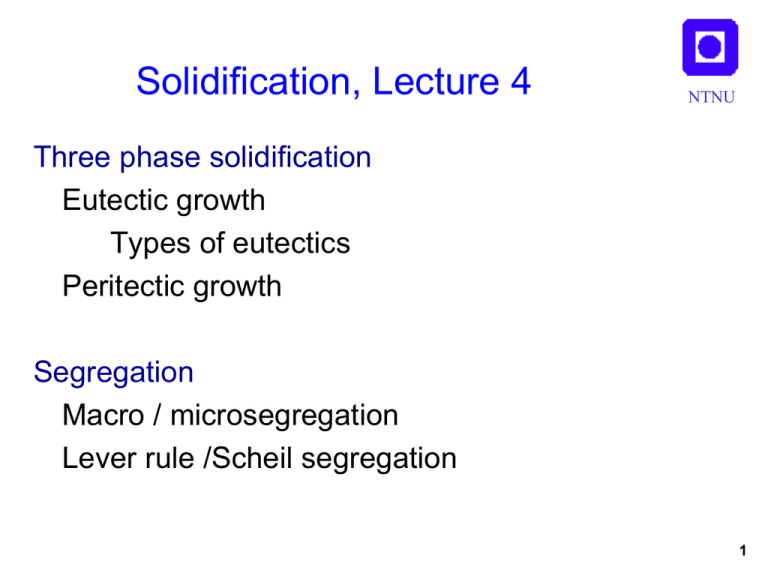
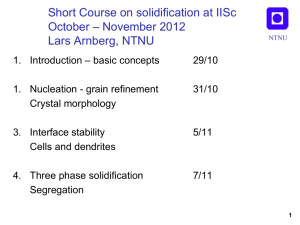
Solidification, Lecture 4 NTNU Three phase solidification Eutectic growth Types of eutectics Peritectic growth Segregation Macro / microsegregation Lever rule /Scheil segregation 1 Three phase solidification NTNU Eutectic l α+β Peritectic l+α β Monotectic l1 α+l2 Ce 2 Eutectic solidification NTNU Fibrous Lamellar Regular Al-Mg Irregular Al-Si Reproduced from:W. Kurz & D. J. Fisher: Fundamentals of Solidification Trans Tech Publications, 1998 3 Eutectic growth NTNU Growth direction •Simultaneous, cooperative growth of 2 or more phases •Diffusion parallel to growth front •Isothermal growth front •Characteristic lamellar spacing, determined by diffusion and curvature V K1 T K 2 T K 3 V 2 4 Irregular eutectics NTNU •One or both phases grows facetted •Non isothermal growth •Grows at high undercooling Al-Si 5 Interface instabilities of eutectics NTNU Off-eutectic Composition 3:rd element Reproduced from:W. Kurz & D. J. Fisher: Fundamentals of Solidification Trans Tech Publications, 1998 6 Coupled Zone NTNU Al70Cu30 33% Reproduced from:W. Kurz & D. J. Fisher: Fundamentals of Solidification Trans Tech Publications, 1998 7 Off eutectic growth 1 NTNU Al-30%Cu Low temperature gradient, high growth rate Dendrites + eutectic 8 Off eutectic growth 2 NTNU Al-30%Cu High temperature gradient, low growth rate Coupled eutectic 9 Off eutectic growth 3 NTNU Al-30%Cu High temperature gradient, high growth rate Cellular eutectic 10 Decoupled, divorced eutectic NTNU •Major phase grows on existing dendrites. •Small amount of eutectic •No cooperative growth Al-5Cu 11 Ternary & higher eutectics NTNU Cooperative growth of 3 or more phases Chinese script Al-Mg-Si 12 Peritectic solidification NTNU Primary: l→α Peritectic: l + α →β Eutectic: l → β+γ Reproduced from:W. Kurz & D. J. Fisher: Fundamentals of Solidification Trans Tech Publications, 1991 13 Peritectic solidification NTNU l + α →β • Occurs at l/α interface • Layer of β envelops α • Further transformation requires solid state diffusion through β • Seldom to completion α β 14 Segregation NTNU Macrosegregation Scale: Casting Microsegregation Scale: Secondary dendrite arm spacing λ2 Reproduced from:M. C. Flemings Solidification Processing Mc Graw Hill, 1974 15 Solute redistribution NTNU •Redistribution of solute since liquid- and solid solubility are different. T l •Liquid enriched in solute (eutectic) Cl Cs •Liquidus temperature decreases. s C0 k=Cs/Cl C •Equilibrium at s/l front but not always in the solid or liquid due to slow diffusion Dl ~10-9 – 10-8 m2/s Ds ~ 10-13 – 10-12 m2/s 16 Complete equilibrium NTNU • Valid only in special cases, slow solidification, fast diffusion • Lever rule: T l Cl Cs s fs fl (Cl - C0 ) = f s (C0 - Cs ) fl C0 C0 Cl = 1- (1- k ) f s C • Solid with uniform composition C0 k=Cs/Cl 17 No solid diffusion, complete mixing in liquid NTNU • Reasonable assumption: slow diff. in solid, faster diffusion, small diffusion distances, and convection in liquid. • Gulliver-Scheil equation T l Cl Cs s Cl C0 (1 f s )(k 1) C0 C • Reasonable predictions in most cases k=Cs/Cl 18 Limited solid diffusion, full liquid mixing • T Back diffusion of solute into solid Cl C0 (1 (1 2k) f s )(k 1)(12k ) l Cl Cs • s Fourier number determines amount of diffusion C0 k=Cs/Cl NTNU C • Ds t f L2 Diffusion distance for dendritic structures L 2 2 19 Segregation with back diffusion NTNU Reproduced from:W. Kurz & D. J. Fisher: Fundamentals of Solidification Trans Tech Publications, 1998 20 Freezing point during segregation NTNU Reproduced from:W. Kurz & D. J. Fisher: Fundamentals of Solidification Trans Tech Publications, 1998 21 Microsegregation NTNU Freezing range, ΔT*, larger than equilibrium freezing range, ΔT0 Liquidus concentration Cl higher than C0/k, often reaches eutectic conc. Ce T l C0 T0 Reproduced from:W. Kurz & D. J. Fisher: Fundamentals of Solidification Trans Tech Publications, 1998 s C0/k ΔT* Ce C 22 Example Segregation in Al-1%Si NTNU How much eutectic? C0 Assume full equilibrium. 660 No eutectic 577 1.65 11.7 23 Example Segregation in Al-1%Si NTNU How much eutectic? Assume no diff in solid, full mixing in liquid C0 660 Assume straight lines k=Cs/Cl=1.65/11.7=0.14 577 1.65 11.7 Cl C0 (1 f s )(k 1) Fraction eutectic, fe= fl=(1-fs)when Cl=Ce fe= 0.06 24 Example Segregation in Al-1%Si NTNU How much eutectic? Assume some diffusion in solid Assume cooling rate 1K/s Assume λ2=60 μm Assume D=10-12m2/s C0 660 Cl C0 (1 (1 2k) f s )(k 1)(12k ) ( k - 1) /(1- 2 a k ) Cl = C0 [1- (1- 2a k ) f s ] 577 1.65 11.7 Ds t f L2 m=dT/dCl=577-660/11.7=-7.1 Liquidus temperature, Tl=Tf+mC0=653C Freezing range, ΔT* : 653-577=76K tf= ΔT* /(dT/dt)=76s L= λ2/2=30μm α=0.08 fe=0.035 25 Summary/ Conclusions NTNU • Eutectic structures can be regular/irregular, depending on facetted or non-facetted growth. • Eutectic structures can be lamellar or fibrous, depending on relative amounts of the phases. • Eutectic grows with phases side by side in a coupled way with diffusion parallel to the front. The front is macroscopically flat and isothermal. • Eutectics are characterized by a lamellar spacing, λ, which is controlled by diffusion and curvature and is a function of growth rate. • Irregular eutectics grow at a higher undercooling and a non-isothermal front. • Alloys with off-eutectic compositions can grow in a coupled way depending on temperature gradient and growth rate • Small amounts of residual eutectics often solidify in a decoupled, “divorced” way 26 Summary / Conclusions NTNU • Peritectic reactions, l + α →β, occur at the interface between α and the melt. This means that α becomes isolated and further reaction can only occur by solid state diffusion through β which is a slow process. 27 Summary/ Conclusions NTNU • Redistribution of solute since liquid- and solid solubility are different leads to segregation. • Microsegregation occurs as concentration variations on a scale of the secondary dendrite arm spacing • Equilibrium solidification only occurs in special cases, fast solid diffusion, slow cooling. • Assumption of no solid diffusion, complete mixing in liquid often realistic assumption • Fourier number used to assess degree of back diffusion into solid during solidification • Non-equilibrium segregation causes freezing range, ΔT* to be larger than equilibrium freezing range, ΔT0 and liquidus concentrationCl to increase beyondC0/k, often up to Ce. 28 NTNU Lars Arnberg Dept of Materials Science and Engineering Norwegian University of Science and Technology 7491 Trondheim, Norway arnberg@ntnu.no Tel: +47 930 03 212 (India: 948 2965 476) 29