IISc solidification lecture 1
advertisement
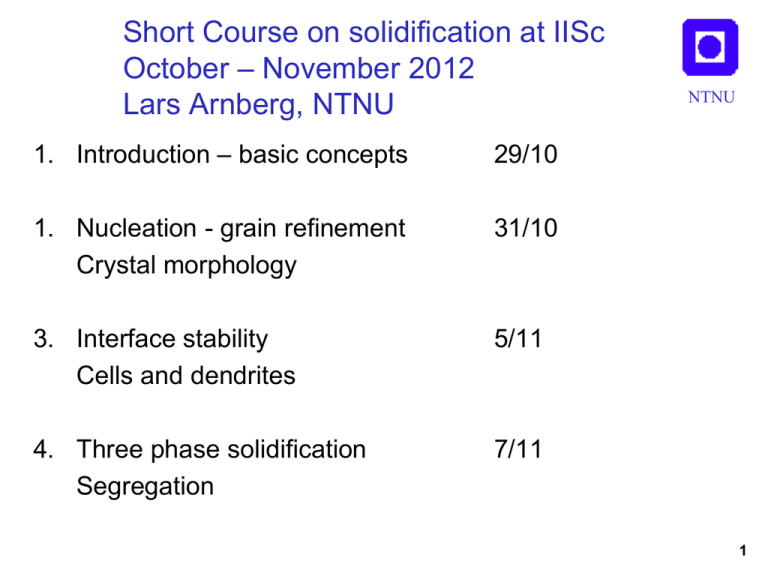
Short Course on solidification at IISc October – November 2012 Lars Arnberg, NTNU 1. Introduction – basic concepts 29/10 1. Nucleation - grain refinement Crystal morphology 31/10 3. Interface stability Cells and dendrites 5/11 4. Three phase solidification Segregation 7/11 NTNU 1 Solidification, Lecture 1 NTNU Introduction / Basic concepts Simple heat flow during solidification Mushy Zone Columnar / equiaxed solidification Curvature effects Phase diagrams – solute redistribution 2 Microstructure NTNU Solidification of metals is a crystallisation process Microstructure development Depends on Microstructure Composition (constitution) Concentration, C Phase diagram, k, m Casting conditions Growth rate, V Temperature gradient, G Cooling rate, G*V Crystal types, phases Crystal morphology Crystal size Chemical composition 3 Microstructure NTNU Increasing concentration Increasing constitutional undercooling (Tc) Increasing morphological instability Increasing cooling rate (G*V) Structure refinement 4 Heat flow NTNU A dT dfs q c H v dt dt dT A dfs H q dt vc dt c Reproduced from:W. Kurz & D. J. Fisher: Fundamentals of Solidification Trans Tech Publications, 1998 5 Mushy zone NTNU Alloys will solidify over a temperature Interval, ΔTf M. Z. is where solidification occurs Depending on freezing range and temp gradient a a Tf G 6 Controlled solidification NTNU a: Bridgman furnace Independent control of G & V. G & V constant b: Directional chill casting G & V time dependant dT/dt = GV s=Kt1/2 Reproduced from:W. Kurz & D. J. Fisher: Fundamentals of Solidification Trans Tech Publications, 1998 7 Growth modes morphology & temperature distribution Directional Growth of columnar crystals NTNU Free growth of equiaxed crystals Pure metal Alloy Reproduced from:W. Kurz & D. J. Fisher: Fundamentals of Solidification Trans Tech Publications, 1998 Positive G Negative G 8 Structure of castings NTNU 9 Capillary effects; solid/liquid interface NTNU • Undercooling T K • Curvature 2/r for sphere dA K= dV • Gibbs Thomson ~ 10-7 Km s f Solidification microstructures given by competition between: •Curvature : tends to maximise scale •Diffusion: tends to minimise scale Reproduced from:W. Kurz & D. J. Fisher: Fundamentals of Solidification Trans Tech Publications, 1998 10 Phase digram, solute redistribution NTNU • Eutectic phase diagram T l C0 Tl T0 Ts s Cs C0 • Lower solubility of alloying elements in s than in l • k=Cs/Cl<1 (distribution coefficient) Cl • m= dTl/dC<0 C • k and m constants if solidus & liquidus lines are straight C0 (1 k) T0 mC0 m k 11 Al-Fe Al-Mg NTNU Al-Si Al-Mn Eutectic Al phase diagrams for important alloying elements 12 Al-Fe k=0.03 Al-Mn k=0.90 AlMg k=0.44 Al-Si k=0.14 NTNU Al phase diagrams with different partition coefficients k=Cs/Cl 13 Summary/ Conclusions NTNU • Solidification is accomplished by external cooling of a melt. Needed for decreasing the temperature and removing latent heat of fusion • Metals solidify at a distinct freezing point, alloys have a solidification interval (freezing range) • Solidification microstructure will depend on both composition, (C0) constitution (k, m) and process (G, V) • Control of V and G will differ between casting processes • Solidification will occur in mushy zone. Extent of MZ will depend on temperature gradient and freezing range • Crystal may grow directionally as columnar grains (G>0) or freely from an undercooled melt as equiaxed grains (G<0) • Creation of s/l interface will require undercooling. ΔTr will increase with increased curvature (small crystal radii) 14 Summary/ Conclusions NTNU • Scale of solidification microstructure will be determined by diffusion (decreasing) and curvature (increasing) • Solidification of alloys means redistribution of solute between s and l. Determined by distribution coefficient, k. 15 Symbols NTNU C: concentration k: distribution coefficient k=Cs/Cl m: liquidus slope, dT/dC V: growth rate m/s T: temperature: K ΔT: undercooling, K q: heat flux W/m2 A: area m2 V: volume m3 t: time, s ΔH: heat of fusion J/m3 c: heat capacity: J/(m3K) fs: fraction solid ΔTf: freezing range, K G: temperature gradient, dT/dx K/m Δsf: entropy of fusion, J/(m3K) σ: solid/liquid interface energy, J/m2 Cl: liquid concentration Cs: solid concentration C0: Initial alloy concentration 16