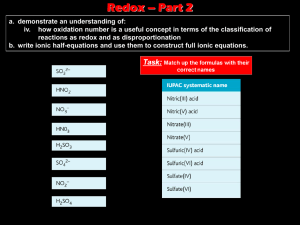
Notes: Oxidation Numbers & Redox Agents
advertisement

Redox Reactions Chapter 18 + O2 Oxidation-Reduction (Redox) Reactions “redox” reactions: rxns in which electrons are transferred from one species to another oxidation & reduction always occur simultaneously we use OXIDATION NUMBERS to keep track of electron transfers Rules for Assigning Oxidation Numbers: 1) the ox. state of any free (uncombined) element is zero. Ex: Na, S, O2, H2, Cl2, O3 Rules for Assigning Oxidation Numbers: 2) The ox. state of an element in a simple ion is the charge of the ion. Mg2+ oxidation of Mg is +2 Rules for Assigning Oxidation Numbers: 3) the ox. # for hydrogen is +1 (unless combined with a metal, then it has an ox. # of –1) Ex: NaOH (H bonded to O) v. NaH (H bonded to Na) H = +1 H = -1 Rules for Assigning Oxidation Numbers: 4) the ox. # of fluorine is always –1. Rules for Assigning Oxidation Numbers: 5) the ox. # of oxygen is usually –2. Why USUALLY? Not -2 when it’s in a peroxide, such as hydrogen peroxide: H2O2 Rules for Assigning Oxidation Numbers: 6) in any neutral compound, the sum of the oxidation #’s = zero. Rules for Assigning Oxidation Numbers: 7) in a polyatomic ion, the sum of the oxidation #’s = the overall charge of the ion. Rules for Assigning Oxidation Numbers: **use these rules to assign oxidation #’s; assign known #’s first, then fill in the #’s for the remaining elements: Examples: Assign oxidation #’s to each element: a) NaNO3 Na = +1 O = -2 Therefore, N = +5 Examples: Assign oxidation #’s to each element: b) SO32+4 -2 O = -2, therefore S must = +4 to balance the charges and have an overall charge of 2- Examples: Assign oxidation #’s to each element: c) HCO3+1 +4 -2 Examples: Assign oxidation #’s to each element: Do on your own: d) H3PO4 e) Cr2O72- f) K2Sn(OH)6 Definitions Oxidation: the process of losing electrons (ox # increases) Reduction: the process of gaining electrons (ox # decreases) Oxidizing agents: species that cause oxidation (they are reduced) Reducing agents: species that cause reduction (they are oxidized) To help you remember… OIL RIG Oxidation Is Loss Reduction Is Gain Are all rxns REDOX rxns? You must determine this… a reaction is “redox” if a change in oxidation # happens; if no change in oxidation # occurs, the reaction is nonredox. Examples MgCO3 is an ionic compound, so what is Mg’s charge in an ionic compound? MgCO3 MgO +2 +4 -2 +2 The carbonate ion CO32is the other ion, let’s figure out C because we already know O. -2 + CO2 +4 -2 Is this a redox or nonredox reaction? NONREDOX (no change in oxidation numbers) Examples Which oxidation numbers do we already know? Zn + CuSO4 ZnSO4 + Cu 0, free element +2 +6 -2 Break down this ionic compound into its ions Cu and SO42So, Cu must be Cu2+ O = -2 in SO42-, so S must be…? +2 +6 -2 0 Is this a redox or nonredox reaction? REDOX reaction Examples NaCl + AgNO3 AgCl + NaNO3 Redox or nonredox? Examples CO2 + H2O C6H12O6 + O2 +4 -2 +1 -2 0 +1 -2 What happened to Carbon? It went from +4 oxidation # to 0. Was Carbon oxidized or reduced? REDUCED OIL RIG (oxidation is losing electrons so oxidation number increases, where as reduction is gaining electrons so oxidation number decreases) 0