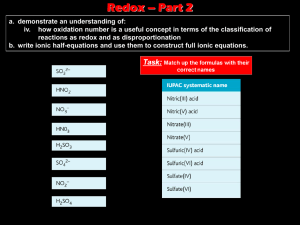
Oxidation Numbers Power point
advertisement

•It is possible to determine the charge of an ion in an ionic compound given the charges of the other ions present in the compound. •Determine the charge on the bromide ion in the compound NaBr given that Na+ has a 1+ charge. •The total charge is 0, so Br- must have a charge of 1in order to balance the 1+ charge of Na+. •Numbers called oxidation numbers can be assigned to atoms in order to keep track of electron distributions in molecular as well as ionic compounds. Oxidation Numbers •The charges on the ions in an ionic compound reflect the electron distribution of the compound. •In order to indicate the general distribution of electrons among the bonded atoms in a molecular compound or a polyatomic ion, oxidation numbers are assigned to the atoms composing the compound or ion. •Unlike ionic charges, oxidation numbers do not have an exact physical meaning; rather, they serve as useful “bookkeeping” devices to help keep track of electrons. Assigning Oxidation Numbers •In general when assigning oxidation numbers, shared electrons are assumed to “belong” to the more electronegative atom in each bond. Shared electrons “belong” to chlorine. •More specific rules are provided by the following guidelines. 1. The atoms in a pure element have an oxidation number of zero. • Examples: all atoms in sodium, Na, oxygen, O2, phosphorus, P4, and sulfur, S8, have oxidation numbers of zero. 2. The more electronegative element in a binary compound is assigned a negative number equal to the charge it would have as an anion. Likewise for the less electronegative element. 3. Fluorine has an oxidation number of -1 in all of its compounds because it is the most electronegative element. 4. Oxygen usually has an oxidation number of -2. • Exceptions: • In peroxides, such as H2O2, oxygen’s oxidation number is -1. • In compounds with fluorine, such as OF2, oxygen’s oxidation number is +2. 5. Hydrogen has an oxidation number of +1 in all compounds containing elements that are more electronegative than it. • It has an oxidation number of -1 with metals. more electronegative metal hydride 6. The algebraic sum of the oxidation numbers of all atoms in a neutral compound equal to zero. 7. The algebraic sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge on the ion. 8. Although rules 1 through 7 apply to covalently bonded atoms, oxidation numbers can also be applied to atoms in ionic compounds similarly. Examples: Assign oxidation numbers to each atom in the following compounds or ions: a. UF6 b. H2SO4 c. ClO3- Solution (a). Start by placing known oxidation numbers above the appropriate elements. From the guidelines, we know that fluorine always has an oxidation number of -1. -1 UF6 Multiply known oxidation numbers by the appropriate number of atoms and place the totals underneath the corresponding elements. There are six fluorine atoms, 6 x -1 = -6. -1 UF6 -6 The compound UF6 is molecular. According to the guidelines, the sum of the oxidation numbers must equal zero. The total of positive oxidation numbers is therefore +6. -1 UF6 +6 -6 Divide the total calculated oxidation number by the appropriate number of atoms. There is only one uranium atom in the molecule, so it must have an oxidation number of +6. +6 -1 UF6 +6 -6 Solution (b). H2SO4 Oxygen and sulfur are each more electronegative than hydrogen, so hydrogen has an oxidation number of +1. Oxygen is not combined with a halogen, nor is H2SO4 a peroxide. Therefore the oxidation number of oxygen is -2. Place these known oxidation numbers above the appropriate symbols. Place the total of the oxidation numbers underneath. +1 -2 H2SO4 +2 -8 The sum of the oxidation numbers must equal zero, and there is only one sulfur atom in each molecule of H2SO4. Because (+2) + (-8) = -6, the oxidation number of each sulfur atom must be +6. +1 +6 -2 H2SO4 +2 +6 -8 Solution (c). ClO3To assign oxidation numbers to the elements in ClO3-, proceed as in parts (a) and (b). Remember, however, that the total of the oxidation numbers should equal the overall charge of the anion, -1. The oxidation numbers of a single oxygen atom in the ion is -2. The total oxidation number due to the three oxygen atoms is -6. For the chlorate ion to have a 1- charge, chlorine must be assigned an oxidation number of +5. +5 -2 ClO3+5 -6 Assign oxidation numbers to each atom in the following compounds or ions: 1.HCl 9.N2O5 2.CF4 10.GeCl2 3.PCl3 11.H2O 4.SO2 12.NO25.HNO3 13.SO426.KH 14.H2CO3 7.P4O10 15.CS2 8.HClO3