Volumetric and Gravimetric analysis with answers
advertisement

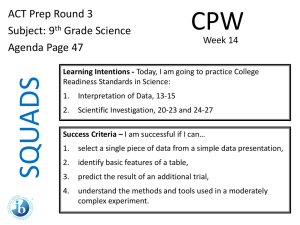
VOLUMETRIC AND GRAVIMETRIC EXAM QUESTIONS Examples of Acids and Bases Strong Acid Hydrochloric HCl Sulphuric H2SO4 Nitric HNO3 (Anything with a high KA value) Weak Acid Ammonium ion NH4 Benzoic C6H5COOH Boric H3BO3 Ethanoic CH3COOH Hydrocyanic HCN (For more weak acids see page 11 of the data book) Strong Base Sodium Hydroxide NaOH Potassium Hydroxide KOH Barium Hydroxide Ba(OH)2 (most hydroxides) Weak Base Ammonia NH3 Alanine C3H5O2NH2 Methylamine CH3NH2 Pyridine C5H5N Titration example 30 mL of 0.10M NaOH neutralised 25.0mL of hydrochloric acid. Determine the concentration of the acid Write the balanced chemical equation for the reaction NaOH(aq) + HCl(aq) -----> NaCl(aq) + H2O(l) Extract the relevant information from the question: NaOH V = 30mL , M = 0.10M HCl V = 25.0mL, M = ? Check the data for consistency NaOH V = 30 x 10-3L , M = 0.10M HCl V = 25.0 x 10-3L, M = ? Titration example continued Calculate moles NaOH n(NaOH) = M x V = 0.10 x 30 x 10-3 = 3 x 10-3 moles From the balanced chemical equation find the mole ratio NaOH:HCl 1:1 Find moles HCl NaOH: HCl is 1:1 So n(NaOH) = n(HCl) = 3 x 10-3 moles at the equivalence point Calculate concentration of HCl: M = n ÷ V n = 3 x 10-3 mol, V = 25.0 x 10-3L M(HCl) = 3 x 10-3 ÷ 25.0 x 10-3 = 0.12M Back Titration example A sample of calcium carbonate is dissolved with 20.00 mL of 0.2254 M hydrochloric acid and the excess acid is titrated with 0.1041 M sodium hydroxide. After dissolution, a mass of 0.2719 g of the calcium carbonate sample requires a titer of 12.39 mL of sodium hydroxide to reach a phenolphthalein end point. Find the %w/w of CaCO3 in the sample. Back Titration continued • Find the total number of moles of HCl added n(HCl total) = C(HCl) X V(HCl) = (0.2254 M)(0.020L) = 0.004508 mole HCl • Find the number of moles of NaOH needed to neutralise the remaining HCl? • n(NaOH) = C(NaOH) X V(NaOH) = (0.1041 N)(0.01239 L) = 0.001290 mole NaOH • How much HCl is consumed by the NaOH titrant? 1:1 ratio in balanced equation therefore n(HCl in aliquot) =n(NaOH) =0.001290 mole HCl Back Titration continued • Find the number of moles of HCl consumed by the CaCO3 n(HCl consumed) = n(HCl total) – n(HCl aliquot) = 0.004508 – 0.001290 = 0.003218 mole • Find the number of moles of CaCO3 in the sample 2HCl(aq) + CaCO3(aq) CaCl2(aq) + H2O(aq) + CO2(g) n(CaCO3) = ½ n(HCl) = ½ x 0.003218 = 0.001609 mole • Find the mass of CaCO3 in the sample m = n X M = 0.01609 X 100.1 = 0.1610g • Find the %w/w in the sample %w/w = m(CaCO3)/m(sample) X 100% =0.1610/0.2719 X 100% = 59.21% w/w CaCO3 in the sample Exam questions on Gravimetric and volumetric analysis The percentage purity of powdered, impure magnesium sulfate, MgSO4, can be determined by gravimetric analysis. Shown below is the method used in one such analysis. Method • 32.50 g of the impure magnesium sulfate is dissolved in water and the solution is made up to 500.0 mL in a volumetric fl ask. • Different volumes of 0.100 M BaCl2(aq) are added to six separate 20.00 mL samples of this solution. This precipitates the sulfate ions as barium sulfate. The equation for the reaction is Ba2+(aq) + SO42–(aq) → BaSO4(s) The precipitate from each sample is filtered, rinsed with de-ionised water and then dried to constant mass. The results of this analysis are shown on the next page. a. Why is it necessary to rinse the precipitate with de-ionised water before drying? b. Explain why the amount of BaSO4(s) precipitated remains constant for the last four samples tested even though more BaCl2(aq) is being added. . c. Calculate i. the amount, in mole, of SO42–(aq) in the 500.0 mL volumetric flask. i. the percentage, by mass, of magnesium sulfate in the powder. ii. . iii. Titration exam question 0.415 g of a pure acid, H2X(s), is added to exactly 100 mL of 0.105 M NaOH(aq). A reaction occurs according to the equation H2X(s) + 2NaOH(aq) → Na2X(aq) + 2H2O(l) The NaOH is in excess. This excess NaOH requires 25.21 mL of 0.197 M HCl(aq) for neutralisation. Calculate i. the amount, in mol, of NaOH that is added to the acid H2X initially. ii. ii. the amount, in mol, of NaOH that reacts with the acid H2X ii. the molar mass, in g mol–1, of the acid H2X. Back Titration exam question





![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)