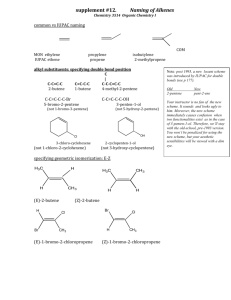
33-Branched Alkanes
advertisement

•Drill: Give the molecular formula & the condensed, skeletal, and complete structures of a hydrocarbon with the same # of carbons as the # of letters in your first name. Branched Alkanes •Any hydrogen in a hydrocarbon chain can be substituted with another hydrocarbon group HHH H-C-C-C-H HHH propane HHH H-C-C-C-H HHH propane HHH H-C-C-C-H H CH H _____propane 3 Alkyl Groups •The hydrocarbon group that replaces the H is called an alkyl group •Suffix: -yl: hydrocarbon group •1 = •2 = •3 = •4 = •5 = Alkyl Groups methyl 6 = ethyl 7= propyl 8 = butyl 9= pentyl 10 = hexyl heptyl octyl nonyl decyl Branched Chains •When an alkane chain gets another hydrocarbon group substituted onto it •Branches: alkyl groups or sidechains Drill: Draw C8H18 using 4 different methods. Branched Chains C C C C C C C C C C Naming Branched Chains Drill: Name Each: H3C-CH2-CH3 C-C-C-C-C 1) Find the longest continuous carbon chain & name it as the main chain. The final name will end with the naming of the main chain • C Hexane •C C C C C C • C C C 2) Number each carbon in the chain; so that, the side chains will be connected to the lowest possible numbers on the carbons • C Hexane 1 2 3 4 5 6 •C C C C C C • C C C 3) Name the side chains or substituted groups, & precede them with the # of the carbon they are connected to & a dash C 2-methyl 1 2 3 4 5 6 C C C C C C C C C 2-methyl 4-ethyl 4) If identical groups appear more than once, use prefixes: di for 2, tri for 3, tetra for 4, penta for 5, etc Examples: dimethyl, tripropyl, etc C 2,2-dimethyl 1 2 3 4 5 6 C C C C C C C C C 4-ethyl 5) Name the sidechains or groups in alphabetical order, but ignore the prefixes when doing so · Examples: ethyl comes before dimethyl, e < m 6) Separate numbers with commas, use hyphens between words & numbers, and write the alkanes as one word · Example: dimethylhexane C CCCCCC C C C 4-ethyl-2,2-dimethylhexane Name the organic compound on the board Drill: Name CH3 CH2 H3C-CH-CH2-CH-CH-CH3 CH3 CH2 CH3 Student Practice Problems 1) H3C-CH2 CH2-CH2-CH3 CH2-CH-CH-CH2-CH-CH2-CH3 CH2-CH2-CH3 1) H3C-CH2 CH2-CH2-CH3 CH3-CH-CH-CH2-CH-CH2-CH3 CH2-CH2-CH3 1) H3C-CH2 CH2-CH2-CH3 CH3-CH-CH-CH2-CH-CH2-CH3 CH2-CH2-CH3 1) H3C-CH2 CH2-CH2-CH3 CH3-CH-CH-CH2-CH-CH2-CH3 CH2-CH2-CH3 6-ethyl-3-methyl-4-propylnonane 2) C C-C C-C-C C-C-C C C C C 2) C C-C C-C-C C-C-C C C C C 2) C C-C C-C-C C-C-C C C C C C 3,4-diethylC-C 2-methyloctane C-C-C C-C-C C C C C 2) 3) 3) 3) 3) 3-ethyl-2,5,7-trimethyl-4,6-dipropyldecane Deriving Structures from Names • Draw the main chain first –Look to the name ending • Draw the branches –Look to the # & group name Draw: • 3,4-diethyl-2-methyloctane • 2,3,5-trimethylhexane • 5-butyl-3-ethyl-2,6,8trimethyl-4-propyldecane Draw: 5butyl-3-ethyl-2,6,8,9tetramethyl-4,7dipropyldecane Drill: Name the following: C-C-C C-C-C C-C-C-C-C-C-C C-C-C-C Draw the Following: • 4,5-diethyl-3,4,5trimethyloctane • 3,3,4,4,5,5-hexaethylheptane Isomers •Compounds that have the same chemical formula, but different shapes Types of Isomers •Structural or skeletal •Geometric or cis/trans •Functional •Positional •Optical Structural Isomers •When there are differences in the carbon chains Structural Isomers C-C-C-C-C C C-C-C-C C C-C-C C Structural Isomers C-C-C-C-C C C-C-C-C C C-C-C C Structural Isomers Pentane methyl butane dimethyl propane Draw & name all the possible structural isomers for C6H14 Draw & name all the possible structural isomers for C8H18 Draw & name all the possible structural isomers for C7H16 Drill: Draw & name all the possible structural isomers for C6H14 This Week’s Schedule • Lab Tomorrow • Review Thursday • Test Friday Name: C-C-C C C-C-C-C-C-C-C C C-C C-C-C C C-C-C-C-C-C-C C C-C C-C-C C C-C-C-C-C-C-C C C-C 3,5-diethyl-3,5dimethyloctane Draw the Following: • 3,5-diethyl-2,4,6trimethyloctane • 5-butyl-3-ethyl-2,6,6,8tetramethyl-4-propylnonane Draw the condensed, complete, skeletal, & stick structures for: •3-ethyl-2,2dimethylpentane Draw & name all the possible structural isomers for C9H20 Drill: Name & describe 5 organic prefixes & 4 organic suffixes Test on Alkanes tomorrow Review Identify •Organic terms •Organic prefixes •Organic suffixes Organic Prefixes •1 = •2 = •3 = •4 = •5 = methethpropbutpent- 6= 7= 8= 9= 10 = hexheptoctnondec- Name: CH3-CH2 H3C-CH-CH2-C-CH2-CH3 CH3-CH2 CH3 CH3-CH2 H3C-CH-CH2-C-CH2-CH3 CH3-CH2 CH3 CH3-CH2 H3C-CH-CH2-C-CH2-CH3 CH3-CH2 CH3 3-ethyl-3,5-dimethylheptane CH3-CH2 H3C-CH-CH2-C-CH2-CH3 CH3-CH2 CH3 Name: C-C C-C C-C-C-C-C-C-C-C-C C CC C C-C C-C C-C-C-C-C-C-C-C-C C CC C C-C C-C C-C-C-C-C-C-C-C-C C CC C 6,7-diethyl-3,4,7-trimethyldecane C-C C-C C-C-C-C-C-C-C-C-C C CC C Name: 5-ethyl-2,4,6,7tetramethylnonane Name: 5-butyl-2,3,7,8-tetramethyl-6-propyldecane Draw the complete structure of: 3-ethyl-2-methylpentane Draw the condensed structure of: 3-ethyl-2,4,5-trimethylhexane Draw a skeletal structure of: Octamethylhexane Draw a stick structure of: 5-butyl-3-ethyl-2,4,6,8tetramethyl-4-propyl decane Draw & Name 10 isomers of: C8H18 Draw an optical isomer of: C C C-C-C-C-C-C-C C