Molarity and Dilutions Power Point
advertisement

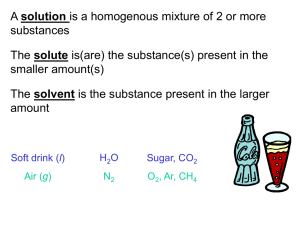
Chemistry: Atoms First Julia Burdge & Jason Overby Chapter 9 .5 Homework: 61, 63, 65, 67, 71, 73, 75, 77, 79 and 81 Kent L. McCorkle Cosumnes River College Sacramento, CA Copyright (c) The McGraw-Hill Companies, Inc. Permission required for reproduction or display. A solution is a homogenous mixture of 2 or more substances The solute is(are) the substance(s) present in the smaller amount(s) The solvent is the substance present in the larger amount Solution Solvent Solute Soft drink (l) H2O Sugar, CO2 Air (g) N2 O2, Ar, CH4 Soft Solder (s) Pb Sn 4.1 Solutions Solvent solute When the solvent is water the solution is said to be aqueous 9.1 General Properties of Aqueous Solutions A solution is a homogenous mixture of two or more substances. The substance present in the largest amount (moles) is referred to as the solvent. The other substances present are called the solutes. A substance that dissolves in a particular solvent is said to be soluble in that solvent. 9.5 Concentration of Solutions Molarity (M), or molar concentration, is defined as the number of moles of solute per liter of solution. molarity = moles solute liters solution Other common rearrangements: L= mol M mol = M L Worked Example 9.8 For an aqueous solution of glucose (C6H12O6), determine (a) the molarity of 2.00 L of a solution that contains 50.0 g of glucose, (b) the volume of this solution that would contain 0.250 mole of glucose, and (c) the number of moles of glucose in 0.500 L of this solution. Strategy Convert the mass of glucose given to moles, and use the equations for Think About Check see that magnitude of your answers interconversions of M,Itliters, andtomoles to the calculate the answers. are logical. For example, the mass given in the problem corresponds 50.0 g to 0.277 mole of solute. If you (b),mol for the moles of glucose = are asked, as in=part 0.277 180.2 g/mol volume that contains a number of moles smaller than 0.277, make sure your answer is smaller than the original volume. Solution 0.277 mol C6H12O6 (a) molarity = = 0.139 M 2.00 L solution 0.250 mol C6H12O6 (b) volume = = 1.80 L 0.139 M solution (c) moles of C6H12O6 in 0.500 L = 0.500 L×0.139 M = 0.0695 mol 4.5 Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution. Dilution Add Solvent Moles of solute before dilution (i) = Moles of solute after dilution (f) MiVi = MfVf 4.5 How would you prepare 60.0 mL of 0.2 M HNO3 from a stock solution of 4.00 M HNO3? MiVi = MfVf Mi = 4.00 Vi = Mf = 0.200 MfVf Mi Vf = 0.06 L Vi = ? L 0.200 x 0.06 = = 0.003 L = 3 mL 4.00 3 mL of acid + 57 mL of water = 60 mL of solution Another Dilution Problem If 32 mL stock solution of 6.5 M H2SO4 is diluted to a volume of 500 mL What would be the resulting concentration? M1*V1 = M2*V2 (6.5M) * (32 mL) = M2 * (500.0 mL) M2 = 6.5 M * 32 mL 500 mL M2 = 0.42 M Concentration of Solutions Dilution is the process of preparing a less concentrated solution from a more concentrated one. moles of solute before dilution = moles of solute after dilution Concentration of Solutions In an experiment, a student needs 250.0 mL of a 0.100 M CuCl2 solution. A stock solution of 2.00 M CuCl2 is available. How much of the stock solution is needed? Solution: Use the relationship that moles of solute before dilution = moles of solute after dilution. Mc × Lc = Md × Ld (2.00 M CuCl2)(Lc) = (0.100 M CuCl2)(0.2500 L) Lc = 0.0125 L or 12.5 mL To make the solution: 1) Pipet 12.5 mL of stock solution into a 250.0 mL volumetric flask. 2) Carefully dilute to the calibration mark. Concentration of Solutions Because most volumes measured in the laboratory are in milliliters rather than liters, it is worth pointing out that the equation can be written as Mc × mLc = Md × mLd Worked Example 9.9 What volume of 12.0 M HCl, a common laboratory stock solution, must be used to prepare 150.0 mL of 0.125 M HCl? Strategy Mc = 12.0 M, Md = 0.125 M, mLd = 250.0 mL Solution 12.0 M × mLc = 0.125 M × 250.0 mL mLc = 0.125 M × 250.0 mL = 2.60 mL 12.0 M Think About It Plug the answer into Equation 9.4, and make sure that the product of concentration and volume on both sides of the equation give the same result. Worked Example 9.10 Starting with a 2.0 M stock solution of hydrochloric acid, four standard solutions (1 to 4) are prepared by sequential diluting 10.00 mL of each solution to 250.00 mL. Determine (a) the concentrations of all four standard solutions and (b) the number of moles of HCl in each solution. Strategy (a) Md = Mc× mLc -1 L ; (b) mol = M×L, 250.00 mL = 2.500×10 mLd Solution 2.00 M × 10.00 mL -2 M (a) Md1 = = 8.00×10 250.00 mL 8.00×10-2 M × 10.00 mL = 3.20×10-3 M Md2 = 250.00 mL 3.20×10-3 M × 10.00 mL Md3 = = 1.28×10-4 M 250.00 mL 1.28×10-4 M × 10.00 mL = 5.12×10-6 M Md4 = 250.00 mL Worked Example 9.10 (cont.) Solution (b) mol1 = 8.00×10-2 M × 2.500×10-1 L = 2.00×10-2 mol mol2 = 3.20×10-3 M × 2.500×10-1 L = 8.00×10-4 mol mol3 = 1.28×10-4 M × 2.500×10-1 L = 3.20×10-5 mol mol4 = 5.12×10-6 M × 2.500×10-1 L = 1.28×10-6 mol Think About It Serial dilution is one of the fundamental practices of homeopathy. Some remedies undergo so many serial dilutions that very few (if any) molecules of the original substance still exist in the final preparation. Worked Example 9.11 Using square-bracket notation, express the concentration of (a) chloride ion in a solution that is 1.02 M in AlCl3, (b) nitrate ion in a solution that is 0.451 M in Ca(NO3)2, and (c) Na2CO3 in a solution in which [Na+] = 0.124 M. Strategy Use the concentration given in each case and the stoichiometry indicated in the corresponding chemical formula to determine the concentration of the specified ion or compound. Solution (a) There are 3 moles of Cl- ion for every 1 mole of AlCl3, AlCl3(s) → Al3+(aq) + 3Cl-(aq) so the concentration of Cl- will be three times the concentration of AlCl3. [Cl-] 3 mol Cl= [AlCl3] × 1 mol AlCl3 1.02 mol AlCl3 3 mol Cl= × L 1 mol AlCl3 3.06 mol Cl= = 3.06 M L