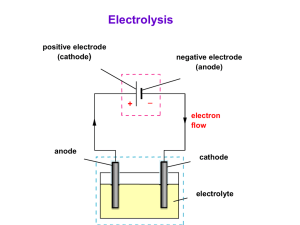
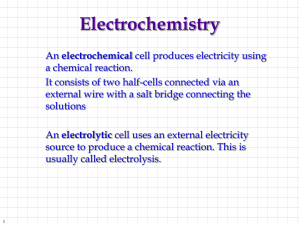
Fe 2+
advertisement

Chapter 20 Electrochemistry and Oxidation – Reduction Mg + HCl → MgCl2 + H2 Mg + HCl → MgCl2 + H2 • Redox Reactions: – Reduction is the decrease of an oxidation number by the gain of electrons. – Oxidation is the increase of an oxidation number by the loss of electrons. Mg + HCl → MgCl2 + H2 LEO the lion says GER Lose Electrons Oxidation Gain Electrons Reduction Mg + HCl → MgCl2 + H2 • Half-Reactions: Oxidation ½ reaction: Mg → Mg2+ + 2eElectron flow Reduction ½ reaction: 2H+ + 2e- → H2 Half-Cells • A half-cell represents a half-reaction from a redox reaction. • A half-cell is made by placing a piece of reactant in an electrolyte solution. • The anode is the half-cell where oxidation occurs. • The cathode is the half-cell where reduction occurs. Mg + HCl → MgCl2 + H2 • Half-Reactions: Oxidation ½ reaction: Mg → Mg2+ + 2e- •Half-Cells: A 1 1 1 A Reduction ½ reaction: 2H+ + 2e- → H2 A 1 1 1 Mg + HCl → MgCl2 + H2 AA 1 1 1 Oxidation (Anode) Mg → Mg2+ + 2e- A A 1 1 AA a Aaaaa 1 Reduction (Cathode) 2H+ + 2e- → H2 Mg + HCl → MgCl2 + H2 Oxidation (Anode) Mg → Mg2+ + 2e- Reduction (Cathode) 2H+ + 2e- → H2 Mg + HCl → MgCl2 + H2 This is a battery. A battery is a type of galvanic cell (voltaic cell). A galvanic cell is a device in which a chemical reaction produces electrical energy. Mg + HCl → MgCl2 + H2 2.37V Oxidation (Anode) Mg → Mg2+ + 2e- Reduction (Cathode) 2H+ + 2e- → H2 Standard Reduction Potentials Figure 20.1 Page 650 Mg + HCl → MgCl2 + H2 E°cell = E°ox + E°red • E°cell = electromotive force (emf) = voltage = cell potential Understanding Reduction Potentials Standard conditions: •1M for ion concentrations •1atm for gas pressures •25°C The standard hydrogen electrode Measuring Potential for a Copper Half-Cell Measuring Potential for a Copper Half-Cell Zn + CuSO4(aq) ZnSO4(aq)+ Cu Zn + CuSO4(aq) ZnSO4(aq)+ Cu • • • • • Write the half-reactions Draw the half-cells Label the anode and the cathode Label the direction of electron flow Draw a salt bridge and label the direction of ion flow within it. • Describe what you would observe at the anode as the reaction proceeds. • Describe what you would observe at the cathode as the reaction proceeds. • Calculate the cell potential (E°cell) Zn + CuSO4(aq) ZnSO4(aq)+ Cu A Zn + CuSO4(aq) ZnSO4(aq)+ Cu E°cell and spontaneity • +E°cell = spontaneous cell reaction • -E°cell = nonspontaneous cell reaction What is the potential for the cell Zn | Zn2+(1.0 M) || Cu2+(1.0 M) | Cu E°cell = +1.10V The reaction is spontaneous Zinc reacts with a solution of copper (II) ions What is the potential for the cell Ag | Ag+(1.0 M) || Li+(1.0 M) | Li • From the table of standard reduction potentials (Appendix H), you find: Ag → Ag+ + eE°ox = -0.7991V Li+ + e- → Li E°red = -3.09V E°cell = -3.8891V E°cell = -3.89V • The reaction is nonspontaneous • Silver will not react with a solution of lithium ions E°red = – 0.440V Write the cell reaction and calculate the potential of this galvanic cell. Co + 2Fe3+ → Co2+ + 2Fe2+ E°cell = +1.048V Write the cell reaction and calculate the potential of a cell consisting of a standard bromine electrode as the anode and a standard chlorine electrode as the cathode. 2Br- + Cl2 → Br2 + 2ClE°cell = +0.2943V Write the cell reaction and calculate the potential of a cell consisting of a standard bromine electrode as the anode and a standard chlorine electrode as the cathode. Free Energy Change and Cell Potential ∆G = ‒nFEcell n = number of moles of electrons F = faraday = 96,485 J V-1 mol-1 ≈ 96,500 J V-1 mol-1 ≈ 96.5 kJ V-1 mol-1 Free Energy Change and Cell Potential ∆G = ‒nFEcell How do ∆G and Ecell relate? Calculate the cell potential and the ∆G for the reaction: Cd + Pb2+ → Cd2+ + Pb A Calculate the cell potential and the ∆G for the reaction: Cd + Pb2+ → Cd2+ + Pb E°cell = +0.277V ∆G = -53.5 kJ Calculate the cell potential and the ∆G for the reaction: Sn4+ + 2Fe2+ → Sn2+ + 2Fe3+ Calculate the cell potential and the ∆G for the reaction: Sn4+ + 2Fe2+ → Sn2+ + 2Fe3+ E°cell = -0.62V ∆G = +120kJ The Effect of Concentration on Cell Potential Ecell vs. E°cell The Nernst Equation (Page 657) Concentration and Ecell • Example. Calculate the cell potential for the following: Fe(s) + Cu2+(aq) Fe2+(aq) + Cu(s) Where [Cu2+] = 0.30 M and [Fe2+] = 0.10 M Concentration and Ecell (cont.) • First, need to identify the 1/2 cells Fe(s) + Cu2+(aq) Fe2+(aq) + Cu(s) Cu2+(aq) + 2e- Cu(s) E°1/2 = 0.34 V Fe2+(aq) + 2e- Fe(s) E°1/2 = -0.44 V Fe(s) Fe(s) + Cu2+(aq) Fe 2+(aq) + 2e- E°1/2 = +0.44 V Fe2+(aq) + Cu(s) E°cell = +0.78 V Concentration and Ecell (cont.) • Now, calculate Ecell Fe(s) + Cu2+(aq) Fe2+(aq) + Cu(s) E°cell = +0.78 V Ecell = E°cell - (0.05916/n)log(Q) Fe (0.10) Q 0.33 Cu (0.30) 2 2 Ecell = 0.78 V - (0.05916 /2)log(0.33) Ecell = 0.78 V - (-0.014 V) = 0.794 V Concentration and Ecell (cont.) • If [Cu2+] = 0.30 M, what [Fe2+] is needed so that Ecell = 0.76 V? Fe(s) + Cu2+(aq) Fe2+(aq) + Cu(s) E°cell = +0.78 V Ecell = E°cell - (0.05916/n)log(Q) 0.76 V = 0.78 V - (0.05916/2)log(Q) 0.02 V = (0.05916/2)log(Q) 0.676 = log(Q) 4.7 = Q Concentration and Ecell (cont.) Fe(s) + Cu2+(aq) Fe2+(aq) + Cu(s) 4.7 = Q Q 2 Fe Cu 2 4.7 Fe Q 4.7 0.30 2 [Fe2+] = 1.4 M Comparing Q and Ecell From the last two problems: E°cell = +0.78V Fe(s) + Cu2+(aq) Fe2+(aq) + Cu(s) [Cu2+] [Fe2+] Q Ecell 0.30M 0.10M 0.33 +0.794V 0.30M 1.4M 4.7 +0.76v Ecell = E°cell - (0.05916/n)log(Q) Example 20.9 Page 657 E°cell = +0.179V E°cell vs K This formula can be derived from the Nernst Equation Example 20.10 Page 659 E°cell from example 20.7 = +0.277V K = 2.31 x 109 Calculate the value of K for the reaction. Br2 + 2Cl– → 2Br – + Cl2 K = 1.12 x 10-10 Comparing K and Ecell from the problems: Reactants Products E°cell K +0.277V 2.31 x 109 -0.2943V 1.12 x 10-10 A Table Page 661 Balancing Redox Reactions (See Notes) • • • The procedure used to balance redox reactions can be broken down into several steps. These steps are: Divide the overall reaction into two half equations. Balance each half equation separately: – – – – • • • • balance the atoms in each half equation balance the electrons in each half equation balance any oxygens by adding H2O to one side of the half equation balance any hydrogens by adding H+ to one side of the half equation Multiply each half equation so that the number of electrons lost and gained balance. Add the half equations together and simplify. If asked to balance the reaction in “basic solution” do so by adding OH- ions. Add enough OH- to neutralize any H+ ions. Make sure to add an equal number of OHions to each side of the equation. Check your final answer to make sure that – there are the same number of atoms of each element on both sides – the net charge is the same on both sides – the coefficients are in the simplest whole number ratio possible Cr3+ + Cl- Cr + Cl2 MnO4- + Fe2+ Mn2+ + Fe3+ MnO4- + 8H+ + 5Fe2+ Mn2+ + 4H2O + 5Fe3+ MnO4- + Fe2+ Mn2+ + Fe3+ (basic solution) MnO4- + 8H+ + 5Fe2+ Mn2+ + 4H2O + 5Fe3+ MnO4- + 4H2O + 5Fe2+ Mn2+ + 8OH- + 5Fe3+ Sn2+ + Fe3+ → Sn4+ + Fe2+ 2+ Sn + 3+ 2Fe → 4+ Sn + 2+ 2Fe Zn + NO3- → Zn2+ + NH4+ 4Zn + 10H+ + NO3- → 4Zn2+ + NH4+ + 3H2O Zn + NO3- → Zn2+ + NH4+ (basic solution) 4Zn + 10H+ + NO3- → 4Zn2+ + NH4+ + 3H2O 4Zn + 7H2O + NO3- → 4Zn2+ + NH4+ + 10OH- Galvanic and Electrolytic Cells • See your notebook for supplemental notes on electrolysis. • Oxidation-reduction or redox reactions take place in electrochemical cells. • There are two types of electrochemical cells. – Spontaneous reactions (+Ecell) occur in galvanic (voltaic) cells. – nonspontaneous reactions (-Ecell) occur in electrolytic cells. Electrolytic Cells • Electrolysis is the process by which an electrical current (voltage) causes a chemical reaction to occur. Electrodes & Charge • The anode of an electrolytic cell is positive (cathode is negative), since the anode attracts anions from the solution. • However, the anode of a galvanic cell is negatively charged, since the spontaneous oxidation at the anode is the source of the cell's electrons or negative charge. The cathode of a galvanic cell is its positive terminal. • In both galvanic and electrolytic cells, oxidation takes place at the anode and electrons flow from the anode to the cathode. Zn + Cu2+ → Cu + Zn2+ Ecell = +1.10V e- A Galvanic Cell Figure 20.21 2Na+ + 2Cl- → 2Na + Cl2 Ecell = - 4.07 V Page 667 Electrolytic Cell Electrolysis of NaCl • Cathode (-): Na+ + e- → Na -2.71 V • Anode (+): 2Cl‾ → Cl2 + 2e-1.36 V 2Na+ + 2Cl‾→ 2Na + Cl2 -4.07V • The battery used to drive this reaction must therefore have a potential of at least 4.07 volts. Electrolysis • For any compound undergoing electrolysis it is the negative ion which is oxidized and the positive ion which is reduced. • Cathode (-): Na+ + e- → Na -2.71 V • Anode (+): 2Cl- → Cl2 + 2e-1.36 V -4.07V Rules for the electrolysis of aqueous solutions. • Rule 1: At the anode the negative ion will be oxidized unless it is easier to oxidize water. Anions that do not oxidize as easy as water are F-, NO3- and SO42-. Rules for the electrolysis of aqueous solutions. • Rule 2: At the cathode the positive ion will undergo reduction unless it is easier to reduce water. Cations that do not reduce as easy as water are the metal cations from groups I and II and Al3+. Rules for the electrolysis of aqueous solutions. • The following half reactions are important for you to know in this process. – Oxidation of water: 2H2O → O2 + 4H+ + 4e– Reduction of water: 2H2O + 2e- → H2 + 2OH‾ – Oxidation of hydroxide: 4OH- → O2 + 2H2O + 4e- Oxidation of water: 2H2O → O2 + 4H+ + 4e- Reduction of water: 2H2O + 2e- → H2 + 2OH‾ Oxidation of hydroxide: 4OH- → O2 + 2H2O + 4e- Electrolysis of Aqueous NaCl • Reduction occurs at the cathode. But, now there are two substances that can be reduced at the cathode: Na+ ions and water molecules. – It is much easier to reduce water than Na+ ions, therefore water will reduce at the cathode. Cathode (-): 2H2O + 2e- → H2 + 2OH‾ • Oxidation occurs at the anode: There are two substances that can be oxidized at the anode: Cl‾ ions and water molecules. – Chloride oxidizes easier than water. Therefore Cl‾ oxidizes at the anode. Anode (+): 2Cl‾ → Cl2 + 2eThe overall reaction for this process is 2H2O + 2 Cl‾ → H2 + Cl2 + 2OH‾ Electrolysis of Aqueous NaCl Figure 20.22 Page 668 Electrolysis of aqueous sodium chloride doesn't give the same products as electrolysis of molten sodium chloride. Electrolysis of aqueous sodium chloride doesn't give the same products as electrolysis of molten sodium chloride. Write the half reaction that occurs at each electrode and the overall balanced equation for the electrolysis of NiBr2(aq). • Anode: 2Br – → Br2 + 2e– • Cathode: Ni2+ + 2e– → Ni • Overall: 2Br – + Ni2+ → Br2 + Ni Write the half reaction that occurs at each electrode and the overall balanced equation for the electrolysis of KOH(aq). • Anode: 4OH– → O2 + 2H2O + 4e– • Cathode: 2H2O + 2e– → H2 +2OH– • Overall: 2H2O → 2H2 + O2 Write the half reaction that occurs at each electrode and the overall balanced equation for the electrolysis of Na2SO4 (aq). • Anode: 2H2O → O2 + 4H+ + 4e– • Cathode: 2H2O + 2e– → H2 + 2OH– • Overall: 2H2O → 2H2 + O2 Notice that a salt bridge is not necessary in an electrolytic cell because you are supplying the electricity rather than needing to have the reaction continue in order to produce the voltage. Uses of Electrolysis • Production of many pure elements. Uses of Electrolysis • These inexpensive decorative carabiners have an anodized aluminum surface that has been dyed and are made in many colors. • Anodizing is an electrolytic process used to increase the thickness of the natural oxide layer on the surface of metal parts. Anodizing increases corrosion resistance and wear resistance, and provides better adhesion for paint primers and glues than bare metal. Uses of Electrolysis • Electroplating is a plating process that uses electrical current to reduce cations of a desired material from a solution and coat a object with a thin layer of the material, such as a metal. Uses of Electrolysis • Electrolysis is also used in the cleaning and preservation of old artifacts. Because the process separates the non-metallic particles from the metallic ones, it is very useful for cleaning old coins and even larger objects. Coulombs • A Coulomb (C) is the amount of electricity carried in 1 second by a current of 1 amp. C=A•s Amperes (Amps) • The amp is a measure of the amount of electric charge passing a point per unit time. A=? • A current of one amp is one coulomb of charge going past a given point per second. Faradays • The faraday (F) is the charge on 1 mole of electrons. • F = mol e− = 96,485C ≈ 96,500C. Amounts of various metals deposited at the cathode by 1 faraday of electricity (Figure 20.27 Page 671) Faradays Law of Electrolysis • The mass of a substance produced or consumed at an electrode during electrolysis is directly proportional to the amount of electricity that passes through the cell. What mass of Cu is produced at the cathode when 1.600A flows for 1.000 hour through a solution of CuSO4? How much time is required to produce 1.000 x 106g of Mg when a current of 1.500 x 105A flows through a solution of MgCl2? Calculate the volume of H2 gas at 25°C and 1.00 atm that will collect at the cathode when an aqueous solution of Na2SO4 is electrolyzed for 2.00 hours with a 10.0-amp current. 4H2O + 4e– → 2H2 + 4OH– or 2H2O + 2e– → H2 + 2OH–