Electrons in Atoms
advertisement

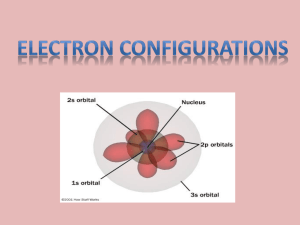
Chapter 5 5.1 The scale model shown is a physical model. However, not all models are physical. In fact, several theoretical models of the atom have been developed over the last few hundred years. You will learn about the currently accepted model of how electrons behave in atoms. 5.1 The Development of Atomic Models ◦ What was inadequate about Rutherford’s atomic model? 5.1 Rutherford’s atomic model could not explain the chemical properties of elements. Rutherford’s atomic model could not explain why objects change color when heated. 5.1 The timeline shows the development of atomic models from 1803 to 1911. 5.1 The timeline shows the development of atomic models from 1913 to 1932. 5.1 The Bohr Model ◦ What was the new proposal in the Bohr model of the atom? 5.1 ◦ Bohr proposed that an electron is found only in specific circular paths, or orbits, around the nucleus. 5.1 Each possible electron orbit in Bohr’s model has a fixed energy. The fixed energies an electron can have are called energy levels or shells. See periodic table A quantum of energy is the amount of energy required to move an electron from one energy level (shell) to another energy level. 5.1 Like the rungs of the strange ladder, the energy levels in an atom are not equally spaced. The higher the energy level occupied by an electron, the less energy it takes to move from that energy level to the next higher energy level. 5.1 The Quantum Mechanical Model ◦ What does the quantum mechanical (electron cloud) model determine about the electrons in an atom? 5.1 ◦ The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations around the nucleus. The exact location of an electron cannot be known with certainty because to do so you would have to interfere with it (Heisenberg Uncertainty Principle). 5.1 Austrian physicist Erwin Schrödinger (1887–1961) used new theoretical calculations and results to devise and solve a mathematical equation describing the behavior of the electron in a hydrogen atom. The modern description of the electrons in atoms, the quantum mechanical model, comes from the mathematical solutions to the Schrödinger equation. 5.1 The propeller blade has the same probability of being anywhere in the blurry region, but you cannot tell its location at any instant. The electron cloud of an atom can be compared to a spinning airplane propeller. 5.1 In the quantum mechanical (electron cloud) model, the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. The cloud is more dense where the probability of finding the electron is high. 5.1 Atomic Orbitals ◦ How do sublevels of principal energy levels differ? 5.1 An atomic orbital is often thought of as a region of space in which there is a high probability of finding an electron. Each energy sublevel corresponds to an orbital of a different shape, which describes where the electron is likely to be found. 5.1 Different atomic orbitals are denoted by letters. The s orbitals are spherical, and p orbitals are dumbbellshaped. 5.1 Four of the five d orbitals have the same shape but different orientations in space. F orbitals are weird. 5.1 The numbers and kinds of atomic orbitals depend on the energy sublevel. The maximum number of electrons per orbital is two. So…. ◦ n = principal energy level ◦ n = the number of sublevels per principal energy level ◦ n2 = the number of orbitals per principal energy level ◦ 2n2 = the number of electrons per principal energy level Make a chart So what is an electron doing in an orbital? ◦ Spinning while moving. Either clockwise or counterclockwise. ◦ Electrons occupying the same orbital spin in opposite directions. Electrons can be described by four quantum numbers: ◦ n= principal quantum number (energy level) ◦ l= orbital quantum number (sublevel) ◦ ml= magnetic quantum number (orbital orientation in space) ◦ ms= spin quantum number (+ or -) No two electrons can have the same four quantum numbers (Pauli Exclusion Principle). 5.2 If this rock were to tumble over, it would end up at a lower height. It would have less energy than before, but its position would be more stable. You will learn that energy and stability play an important role in determining how electrons are configured in an atom. 5.2 Electron Configurations ◦ What are the three rules for writing the electron configurations of elements? 5.2 The ways in which electrons are arranged in various orbitals around the nuclei of atoms are called electron configurations. Three rules—the aufbau principle, the Pauli exclusion principle, and Hund’s rule—tell you how to find the electron configurations of atoms. 5.2 ◦ Aufbau Principle According to the aufbau principle, electrons occupy the orbitals of lowest energy first. In the aufbau diagram below, each box represents an atomic orbital. 5.2 ◦ Pauli Exclusion Principle According to the Pauli exclusion principle, an atomic orbital may describe at most two electrons. To occupy the same orbital, two electrons must have opposite spins; that is, the electron spins must be paired. 5.2 ◦ Hund’s Rule Hund’s rule states that electrons occupy orbitals of the same energy in a way that makes the number of electrons with the same spin direction as large as possible. The principle energy level is the first number, the sublevel is the letter (s,p,d or f) and the number of electrons in the sublevel is the superscript. Number of electrons in sublev Principal energy level 3p4 Type of sublevel 5.2 Orbital Filling Diagram 5.2 Exceptional Electron Configurations ◦ Why do actual electron configurations for some elements differ from those assigned using the aufbau principle? 5.2 ◦ Some actual electron configurations differ from those assigned using the aufbau principle because half-filled sublevels are not as stable as filled sublevels, but they are more stable than other configurations. 5.2 Exceptions to the aufbau principle are due to subtle electron-electron interactions in orbitals with very similar energies. Copper has an electron configuration that is an exception to the aufbau principle. 5.3 Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the gas glow with its own characteristic color. You will learn why each gas glows with a specific color of light. 5.3 Light ◦ How are the wavelength and frequency of light related? 5.3 The amplitude of a wave is the wave’s height from zero to the crest. The wavelength, represented by (the Greek letter lambda), is the distance between the crests. 5.3 The frequency, represented by (the Greek letter nu), is the number of wave cycles to pass a given point per unit of time. The SI unit of cycles per second is called a hertz (Hz). 5.3 The product of the frequency and wavelength always equals a constant (c), the speed of light. 5.3 The wavelength and frequency of light are inversely proportional to each other. 5.3 According to the wave model, light consists of electromagnetic waves. Electromagnetic radiation includes radio waves, microwaves, infrared waves, visible light, ultraviolet waves, X-rays, and gamma rays. All electromagnetic waves travel in a vacuum at a speed of 2.998 108 m/s. 5.3 Sunlight consists of light with a continuous range of wavelengths and frequencies. When sunlight passes through a prism, the different frequencies separate into a spectrum of colors. In the visible spectrum, red light has the longest wavelength and the lowest frequency. 5.3 The electromagnetic spectrum consists of radiation over a broad band of wavelengths. 5.3 Atomic Spectra ◦ What causes atomic emission spectra? 5.3 ◦ When atoms absorb energy, electrons move into higher energy levels. These electrons then lose energy by emitting light when they return to lower energy levels. 5.3 A prism separates light into the colors it contains. When white light passes through a prism, it produces a rainbow of colors. 5.3 When light from a helium lamp passes through a prism, discrete lines are produced. 5.3 The frequencies of light emitted by an element separate into discrete lines to give the atomic emission spectrum of the element. Mercury Nitrogen 5.3 An Explanation of Atomic Spectra ◦ How are the frequencies of light an atom emits related to changes of electron energies? 5.3 In the Bohr model, the lone electron in the hydrogen atom can have only certain specific energies. When the electron has its lowest possible energy, the atom is in its ground state. Excitation of the electron by absorbing energy raises the atom from the ground state to an excited state. A quantum of energy in the form of light is emitted when the electron drops back to a lower energy level. 5.3 ◦ The light emitted by an electron moving from a higher to a lower energy level has a frequency directly proportional to the energy change of the electron. Therefore each transition produces a line of a specific frequency in the spectrum. 5.3 The three groups of lines in the hydrogen spectrum correspond to the transition of electrons from higher energy levels to lower energy levels. 5.3 Quantum Mechanics ◦ How does quantum mechanics differ from classical mechanics? 5.3 In 1905, Albert Einstein successfully explained experimental data by proposing that light could be described as quanta of energy. The quanta behave as if they were particles. Light quanta are called photons. In 1924, De Broglie developed an equation that predicts that all moving objects have wavelike behavior. 5.3 Today, the wavelike properties of beams of electrons are useful in magnifying objects. The electrons in an electron microscope have much smaller wavelengths than visible light. This allows a much clearer enlarged image of a very small object, such as this mite. 5.3 ◦ Classical mechanics adequately describes the motions of bodies much larger than atoms, while quantum mechanics describes the motions of subatomic particles and atoms as waves. 5.3 The Heisenberg uncertainty principle states that it is impossible to know exactly both the velocity and the position of a particle at the same time. This limitation is critical in dealing with small particles such as electrons. This limitation does not matter for ordinary-sized object such as cars or airplanes. 5.3 The Heisenberg Uncertainty Principle