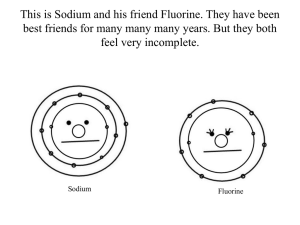
Lewis Dot Structures
advertisement

Electron Arrangements Lewis Dot Structures Electron Arrangements Learning Objectives • Express the arrangement of electrons in atoms using Lewis valence electron dot structures Lewis Dot Structures • Valence electron – an electron in an atom’s highest occupied energy level – Outermost electrons of the electron cloud – Establish chemical characteristics of elements – Only electrons shown in Lewis electron dot structures • Symbolized as dots Lewis Dot Structures • Number of valence electrons is the same as the group number for representative elements Lewis Dot Structures • Helium is an exception because its highest occupied energy level can only hold two electrons Lewis Dot Structures • Number of valence electrons determines behavior – Elements in the same group have similar chemical properties Drawing an Electron Dot Structure Step 1 Identify the symbol for the element and its number of valence electrons using the periodic table. Step 2 Place the corresponding number of electron dots around the symbol. Imagine that the symbol has four sides: top, right, bottom, and left. Begin by assigning one dot per side, moving clockwise around the symbol. Then, if there are still more dots to assign, start adding a second dot to each side until all of the valence electrons have been accounted for. Drawing an Electron Dot Structure Ex) Draw the electron dot structure for phosphorus. Step 1 Identify the symbol for the element and its number of valence electrons using the periodic table. The symbol for phosphorus is P. Phosphorus is in group 5A so it has 5 valence electrons. Step 2 Place the corresponding number of electron dots around the symbol. Imagine that the symbol has four sides: top, right, bottom, and left. Begin by assigning one dot per side, moving clockwise around the symbol. Then, if there are still more dots to assign, start adding a second dot to each side until all of the valence electrons have been accounted for. Does the result make sense? Yes. Electron Arrangements Learning Objectives • Express the arrangement of electrons in atoms using Lewis valence electron dot structures