Atomic mass

Notes #5
I. Parts of an atom
A.
B.
C.
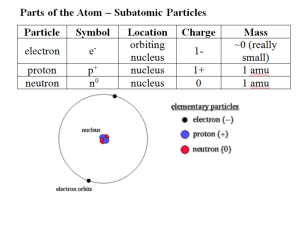
P rotons – positive charge
N eutrons – no charge
E lectrons – negative charge
1. in constant motion around nucleus of atom; travel in orbitals, or shells
II. Determining # of each subatomic particle
Atomic number - # of protons in the nucleus of an atom
Atomic mass - # of protons and neutrons in the nucleus
21
Example for Scandium atom
Sc atomic # = # of p +
45.0
= ___
# p + = # e = ___ atomic mass – atomic # = n 0 = ___
II. Determining # of sub. Part.
• Atomic number - # of protons in the nucleus of an atom
• Atomic mass - # of protons and neutrons in the nucleus
21
• Example for Sc atom
Sc
45.0
atomic # = # of p + = 21
# p + = # e = 21 atomic mass – atomic # = n 0 = 24
III. Atomic Number
Identity of each element is determined by the number of protons in the nucleus.
In a neutral atom, the number of electrons equals the number of protons.
Practice problems pg. 99
IV. Isotopes
A. Atoms of the same element having different atomic masses.
B. Atoms of the same element having different numbers of neutrons.
Hydrogen has three isotopes:
Protium – 0 neutrons
Deuterium – 1 neutron
Tritium – 2 neutrons
IV. Isotopes
C. Naming
It is often important to identify one isotope from another. This is done in two ways.
1. Hyphen notation . This gives the element name (which you can use to determine the atomic number) followed by the atomic mass which you can use to determine the number of neutrons.
Hydrogen – 2
2. Nuclide or nuclear symbol notation
Atomic mass
Atomic number
4
2
He
Practice problems find p
+
, e
-
, n
0
1. Symbol notation 107 Ag
2. Hyphen notation
Potassium – 39
Potassium – 40
Potassium – 41
Solve practice problems pg. 99,101 #’s11-
14. For #14 add on hyphen notation and symbol notation
V. Average Atomic Mass
3.An average sample of hydrogen in nature contains 99.985% protium,
0.015% deuterium and a miniscule amount of tritium.
Protium has an atomic mass of
1.007825 amu.
Deuterium has an atomic mass of
2.014102 amu.
Average Atomic Mass
Multiply the masses of each isotope by its percentage. These are weighted values.
Add all of these values together.
(Do NOT divide by the number of isotopes. This is unnecessary.)
Average Atomic Mass
1.007825 * .99985 = 1.0076738 amu protium
.00015 * 2.014102
= 0.0003021 amu deuterium
1.0076738 + 0.0003021 = 1.00798 amu
1.00798 amu is a weighted average because most of the hydrogen is protium.
Average Atomic Mass
1.00798 amu is a weighted average because most of the hydrogen is protium.
Multiply the masses of each isotope by its percentage. These are weighted values.
Add all of these values together. (Do NOT divide by the number of isotopes. This is unnecessary.)
Average Atomic Mass
The normal way to determine the average mass of something would be to add the two masses and simply divide by two.
Average Atomic Mass
The normal way to determine the average mass of something would be to add the two masses and simply divide by two.
1.007825 + 2.014102 = 3.021927
3.071927 / 2 = 1.5109635 amu
Sample Problem
4. What is the atomic mass of silicon if 92.21% of its atoms have mass 27.977u, 4.70% have mass 28.976 u and 3.09% have mass
29.974?
28.1 u
.9221 x 27.997 = 25.82 u
.0470 x 28.976 = 1.36 u
.0309 x 29.974 = 0.926 u
Sample #2
5. Neon has two isotopes. Neon-20 has a mass of 19.992 u and neon-22 has a mass of
21.991 u. In any sample of 100 neon atoms,
90 will be neon-20 and 10 will be neon-22.
Calculate the average atomic mass of neon.
20.192 u
(.90 x 19.992) + (.10 x 21.991)
17.9928 + 2.1991