Scientific Notation, Accuracy & Significant Figures in Chemistry
advertisement

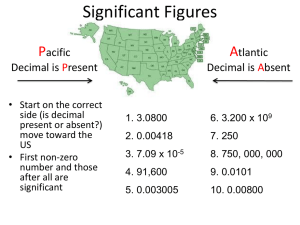
Scientific Notation • Converting into Sci. Notation: – Move decimal until there’s 1 digit to its left. Places moved = exponent. – Large # (>1) positive exponent Small # (<1) negative exponent – Only include sig figs. 65,000 kg 6.5 × 104 kg Scientific Notation Practice Problems 1. 2,400,000 g 2.4 106 g 2. 0.00256 kg 2.56 10-3 kg 3. 7 10-5 km 0.00007 km 4. 6.2 104 mm 62,000 mm Calculating with Scientific Notation Example: (5.44 × 107 g) ÷ (8.1 × 104 mol) = Type on your calculator: 5.44 EXP EE 7 ÷ 8.1 EXP EE 4 EXE ENTER = 671.6049383 = 670 g/mol = 6.7 × 102 g/mol Chemistry Math Accuracy and Precision Percent Error Significant Figures A. Accuracy vs. Precision • Accuracy – a measure of how close a measurement comes to the true value • Precision – a measure of how close a series of measurements are to one another. ACCURATE = CORRECT PRECISE = CONSISTENT Example: Low accuracy – high precision High accuracy – low precision High accuracy – high precision B. Calculating Percent Error • Error – the difference between the experimental value and the accepted value. % error experiment al accepted accepted your value true value 100 B. Percent Error • A student determines the density of a substance to be 1.40 g/mL. Find the error and the % error if the accepted value of the density is 1.36 g/mL. SIGNIFICANT FIGURES A New Way To Count Numbers C. Significant Figures • Indicates the precision of a measurement. • Recording Sig Figs – Sig figs in a measurement include the known digits plus a final estimated digit 2.35 cm 2.83 cm 4.50 cm 28.5 ooCC 30.0 mL 4.29 mL 15.0 C. Significant Figures • Counting Sig Figs –Count all numbers EXCEPT: »Leading zeros -- 0.0025 »Trailing zeros without a decimal point -- 2,500 C. Significant Figures Counting Sig Fig Examples 1. 23.50 4 sig figs 2. 402 3 sig figs 3. 5,280 3 sig figs 4. 0.080 2 sig figs C. Significant Figures • Calculating with Sig Figs – Add/Subtract - The # with the lowest decimal value determines the place of the last sig fig in the answer. 3.75 mL + 4.1 mL 7.85 mL 7.9 mL 224 g + 130 g 354 g 350 g C. Significant Figures • Calculating with Sig Figs – Multiply/Divide - The # with the fewest sig figs determines the # of sig figs in the answer. (13.91g/cm3)(23.3cm3) = 324.103g 4 SF 3 SF 3 SF 324 g EXACT NUMBERS 1. Exact numbers have infinite number of significant figures. Exact numbers include 1. Counting of anything : 22 people or 10 meter sticks, 6 eggs etc. 2. Defined quantities : 12 = 1 doz. 2. Exact numbers do not affect the rounding to the correct number of significant figures or in the calculations of the measurements. C. Significant Figures Practice Problems 1. (15.30 g) ÷ (6.4 mL) 4 SF 2 SF = 2.390625 g/mL 2.4 g/mL 2 SF 2. 18.9 g - 0.84 g 18.06 g 18.1 g Practice Problems (pg. 70 &71). • 3). 61.2 meters + 9.35 meters + 8.6 meters = • 4). 8.3 meters x 2.22 meters= • 5). 8.345 g / 10.6 mL =