Multiplying and Dividing Sig Figs
advertisement

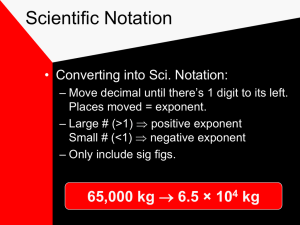
Unit 2: Basic Math for Chemistry Lesson 1: Significant Figures Do Now: Express 44,300 in scientific notation. Book Section: 3.1 Accuracy is how close a measurement is to the “correct” value. Precision is how close measurements are to each other. Accuracy vs. Precision Accuracy vs. Precision In science, measurements have precision, based on the tools we use. The precision of the numbers is determined by the number of significant figures there are. Significant Figures All non-zeroes are significant (1-9). ◦ 423 Zeroes in the beginning… NEVER!! ◦ 0.124 ◦ 0.000000053 Zeroes in the middle… ALWAYS!! ◦ 1204 ◦ 6.0043 ◦ 0.00809 Zeroes at the end… SOMETIMES!! (if there’s a decimal point) ◦ 12400 ◦ 1.2400 ◦ 30400. Counting Significant Figures 28.6 g 3400. cm 910 m 0.04604 L 0.0067000 kg How Many Sig Figs? Your answer will be rounded to the place value of the least precise of the original numbers. 25.1 g + 2.03 g ◦ 25.1 is precise to the tenths place ◦ 2.03 is precise to the hundredths place ◦ Your answer needs to be rounded to the tenths place. Adding and Subtracting Sig Figs 2.099 g + 0.05681 g 5.44 m – 2.6103 m Practice - Adding and Subtracting Sig Figs 2.099 g + 0.05681 g = 2.156 g 5.44 m – 2.6103 m = 2.83 m DON’T FORGET UNITS!!!! Practice - Adding and Subtracting Sig Figs Your answer will be rounded to the same number of sig figs as the lowest number of sig figs by an original number. 3.05 g / 8.5 mL ◦ 3.05 g – 3 sig figs ◦ 8.3 mL – 2 sig figs – answer gets rounded to 2 sig figs 0.36 g/mL Remember...treat units like algebra ◦ Grams divided by milliliters = g/mL Multiplying and Dividing Sig Figs 2.4 g/mL x 15.82 mL 16.25 g / 5.1442 mL Multiplying and Dividing Sig Figs 2.4 g/mL x 15.82 mL = 38 g 16.25 g / 5.1442 mL = 3.159 g/mL Multiplying and Dividing Sig Figs This Week ◦ Thursday – The Metric System (3.2) – HW 2-1 Due ◦ Friday – Pd. 4 Only – Dimensional Analysis (3.3) – HW 2-2 Due ◦ VIDEO: Drew Brees vs. Archer HW 2-1 Due Thursday