Chp 3 Notes
advertisement

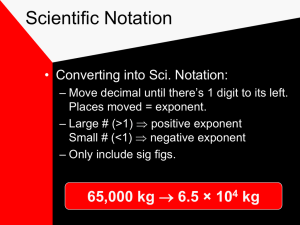
Ch. 3, Scientific Measurement Measurement • Measurement: A quantity that has a number and a unit. Like 52 meters. Scientific Notation • Writing long numbers as smaller ones to a power of ten. • 1,400,000 = 1.4 x 106 = 1.4E6 • .00056 = 5.6 x 10 —4 = 5.6E-4 • 15,093,000 = 1.5093 x 107 = 1.5093E7 Accuracy vs. Precision • *Accuracy: How close a measurement comes to the correct value. • *Precision: How close a series of measurements are to one another. Determining Error of a measurement • Error = Experimental Value - Accepted Value – Can be positive or negative • Percent Error = error / accepted value * 100% – or – Always positive • Example, you measured 99 cm, but it is 100 cm. • Significant Figures: The digits in a measurement that are known plus one that is not known. – Filled 43.1 mL Rules for Sig Figs • Non-Zero Numbers are always significant – Ex) .236 m has 3 sig figs. • Zeros between non-zero numbers are significant. – Ex) 7003 m and 40.79 m each have 4 sig figs. • Leftmost zeros are not significant – Ex) .000099 m, 0.42 m, and .073 m each have 2 sig figs. • Rightmost zeros are significant if they come before or after a period. – Ex) 9000. m, 90.00 m, and 9.090 m each have 4 sig figs. Rules for Sig Figs • Rightmost zeros with no decimal points are no significant. • Ex) 300 m has 1 sig fig. • Exactly defined quantities have an unlimited number of sig figs. • Seen most when converting between measurements. • 60 min = 1 hour, 100 cm = 1 m each have an unlimited number of sig figs. Is a Zero a Significant Number or Not? • • • • 504 L .06 mL 50.0 m 7,000 km Sig Figs in Calculations: • Calculations cannot be more precise than the least precise measurement. • Addition or subtraction - Round the final answer to the same number of decimal places as the measurement with the least number of decimal places. – the last “shared column”. • 13.4 m + 5.254 m = 18.7 m Sig Figs in Calculations: • Multiplication or Division - The final answer should have the same number of sig figs as the measurement that has the least number of sig figs. • 2.4526 m x 8.4 m = 21 m • In this class, delay rounding until the very end. • (3.449L + 6.57L) * 8.779L / 5.2L = 17L International System of Units • How much of something you have is given by Units. Like centimeters, kilometers, etc. • SI is the standard measurement system for science. • • • • • • • * The five SI units used most by chemists are: Distance = meter (m) Mass = kilogram (kg) Temperature = kelvin (K) Time = second (s) Volume = liter (L) Amount = mole (mol). • Prefixes can be used in front of units for powers of 10. • Mass = how much “stuff” is in something. How much matter is in something. • Weight = the pull on something by gravity. • If I go to the moon, which changes and which stays constant? -Weight changes, mass stays the same. Temperature: • Celsius and Kelvin are units of temperature that are the same, just shifted. • K = C + 273 • Water freezes at 0 C and at 273 K. • Absolute Zero = 0 K; a K value will never be negative. *Energy • * Energy - The ability to do work or produce heat. • Joule (J) is the SI unit of energy. • Calorie (cal) is another unit of energy. • 1 J = .239 cal Conversions Convert 7 mm to m. Convert 12.4 Mm to cm. • Convert 7 km/ms to m/s • An experiment asks students to measure 1.50 g of Cu wire. All we have is a 40.0 g spool of Cu. How many students can do the experiment? *Density • *Density = mass / volume • What is the density of 2 g of salt in a volume of 4 L? • What is the density in g/L of 5 kg of salt in a volume of 6 mL? If silver has a density of 10.5 g/L, what is the volume of a silver coin that has a mass of 14 g? • Density decreases as temperature increases. • D=m/v • As T increases, V increases. • Therefore, density decreases. Example Problems!!! • 1. How many sig figs in: 1.480*105 .00070 1.000080 3.00E3 • 2. Perform these calculations and come up with answers that have the correct number of sig figs: 5.667 + .34 5.007E3 / 1.20 4.3 - 1.3590 5.309E3 * 3 • 3. Convert 3.4 ng into kg. • 4. Convert 7E3 cm into Mm. • 5. What is the mass if a substance with a density of 4.5 g/L has a volume of 3 L? 6. How many sig figs in: 1010 3000 3000. 3000.0 7. Convert 3 kg into g. Convert 34 cm into m. Convert 4 m into cm. 8. You have a 4 kg box. You measure it to be 5.8 kg. What is the error? The % error? 9. What is the difference between mass and weight? Accuracy and precision? • 11. Put 5,009,000,000 into Scientific Notation • 12. Put 0.00007890 into Scientific Notation. • 13. Put 8.7 * 10-4 into a regular number. • 14. Which is the largest amount? • A. 2*102 g B. 30 kg C. 45 mg D. 190 g • 15. Convert 40 Kelvin into Celsius • 16. Convert 99 Celsius into Kelvin.