Test
advertisement

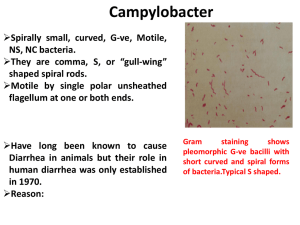
Psuedomonaceae Gram Negative rods Nonfermentative (Strict aerobic) Oxidation of sugars Cytochrome C oxidase Motile Pseudomonas aeroginosa (Piocianic bacillus) Growth in soil and water containing only traces of nutrients. A remarkable ability to withstand disinfectants (found in soap solutions, in antiseptics and in detergents). Persistent in the hospital environment An important role in hospital-acquired infections P.a. Producing 2 pigments: Pyocyanin (colors the pus in a wound blue-green) Pyoverdin /Fluorescein (a yellow-green pigment that fluoresces under ultraviolet light In the lab, these pigments diffuse into the agar, imparting a blue-green color that is useful in identification of the species. In cystic fibrosis patients, P. aeroginosa has a slime layer (glycocalyx): very mucoidal colonies. The slime layer mediates adherence of the organism to mucous membranes of the respiratory tract and prevents antibody from binding to the organism. Pathogenesis Virulance factors: Endotoxin Exotoxin A (inhibits eukaryotic protein synthesis by the same mechanism as diphteria exotoxin) An opportunistic pathogen when neutrophil counts is below 500/uL In those with extensive burns (skin host defenses are destroyed) In those with chronic respiratory disease (such as cystic fibrosis) 10-20% of hospital-acquired infections. In immunosuppressed In those with catheters Clinical finding Can cause infections virtually anywhere in the body, but more frequent in: Urinary tract infections (UTIs) Pneumonia External otitis Wound infections (especially burns). Sepsis with mortality rate of over 50%. Epidemiology 10% of people carry it in the normal flora of the colon and on the skin in moist areas. It can colonize the upper respiratory tract of hospitalized patients. Its ability to grow in simple aqueous solutions has resulted in contamination of respiratory therapy and anesthesia equipments, and even distilled water. Lab diagnosis Non-lactose-fermenting (colorless) colonies on MacConkey or EMB agar. Blue-green pigment on nutrient agar Catalaze and gelatinase positive In TSI: Alkalin/Alkalin Gram negative rods Oxidase-positive Fruity aroma Oxidase Test Detecting cytochrome C oxidase enzyme Indicator: 1% tetra methyl-para-phenylene diamine dihydrochloride Treatment Resistant to many antibiotics Antibiogram test is essential Usually is chosen from penicillins or cephalosporins along with an aminoglycoside. Prevention Keeping neutrophil counts above 500/uL Removing indwelling catheters promptly Taking special care of burned skin Brucella Microbiology characteristics Small bacilli Gram negative Aerobic Capsule Nonmotile Co2 needed Fastidious Virulence factors Endotoxin No exotoxin The organism is an obligative intracellular parasite. Transmission A zoonotic organism From domestic animals: B. melitensis from goat B. abortus from cow B. suis from pig B. canis from dog Entering portals: Mouth, conjunctive, respiratory tract, abraded skin. Pathogenesis Entering the body through ingestion / skin / mucosa Localization in mononuclear phagocytes to the reticuloendothelial system: lymph nodes, liver, spleen, and bone marrow Small granulomas reveal a mononuclear response Effective host defense depends primarily on cellmediated immunity. Some organisms survive within macrophages. The host responses by granulomatous along with lymphocytes and epithelioid giant cells, which can progress to form focal abscesses and caseation. Clinical findings Enlarged lymph nodes, liver and spleen The onset may be insidious or abrupt. Undulant (rising and falling )fever Subclinical infection is common Sweating, weakness and fatigue Incubation period: 2-4 weeks Severe limb and back pains Influenza like onset B. melitensis infections tend to be more severe and prolonged, whereas those caused by B. abortus are more self-limited. Osteomyelitis is the most frequent complication. In untreated cases, symptoms may continue for 2-4 weeks. Most patients recover entirely within 3-12 months but some develop complications marked by involvement of various organs. Laboratory diagnosis Diagnosis can be made clinically if there is a history of exposure. Recovery of the organism requires the use of enriched culture media and incubation in 10% co2. Blood cultures are positive in early disease, but serology is the mainstay of diagnosis. Interpretation is complicated by subclinical infections and persistent antibodies. Treatment Doxycycline Streptomycin Rifampin Control Pasteurizing milk Eradicating infection from herds by immunization of animals and slaughtering of infected animals. Using safety precautions (protective clothing and laboratory safety). Campylobacter Microbiology properties Curved (comma- or S-shaped) Gram-negative rods Microaerophilic (growing in 5% oxygen) Nonfermenting Motile(darting motility) with single flagellum Oxidase positive Important species Campylobacter jejunui : Gastroenteritis Campylobacter fetus: An opportunistic organism Bacteremia in compromised hosts and self-limited diarrhea in previously healthy individuals. Campylobacter coli: Gastroenteritis • Campylobacter jejuni Virulence factors Endotoxin Enterotoxin that acts in the same manner as cholera toxin Transmission and Epidemiology Source of the organisms: Domestic animals, such as cattle, chickens and dogs Person-to-person transmission: oral-fecal The major cause of bacterial diarrhea in developed countries (4.6% of patients with diarrhea, compared with 2.3 and 1% for salmonella and Shigella) Campylobacter jejuni and C. coli are endemic worldwide and hyperendemic in developing countries. Infant and young adults are most often infected. The incidence peak in the summer. Sporadic outbreaks are associated with contaminated animal products or water. Pathogenesis Invasion to the epithelial cells and colonization the small and large intestines often occurs, accompanied by blood in stool causing inflamatory diarrhea and fever. Systematic infections, eg, bacteremia, occur most often in neonates or debilitated adults. Clinical findings Enterocolitis, begins as watery, fuel-smelling diarrhea followed by bloody stools accompanied by fever and severe abdominal pain. Systemic infections, most commonly bactermia, are caused by C. fetus showing symptoms of fever and malaise. Detection of C jejuni and related enteric bacteria. Laboratory diagnosis for C. jejuni A stool specimen Blood agar culture Incubation at 42c in a microaerophilic atmosphere (5% O2 and 10% CO2) Skirrow’s medium (containing vancomycin, trimethoprim, cephalothin, polymyxin, and amphotericin B.) Laboratory diagnosis for C. jejuni Failure to grow at 25 C Oxidase positive Sensitivity to nalidixic acid The identification of C. fetus is confirmed by: Failure to grow at 42 C Ability to grow at 25 C Resistance to nalidixic acid Treatment Erythromycin in C. jejuni enterocolitis An aminoglycoside in C. fetus bacteremia Prevention No vaccine Proper sewage disposal Personal hygiene (hand washing) Helicobacter pylori Multiple flagella Urease Helicobacter pylori Microbiology properties Motile(darting motility) with lophotrichous flagellum Microaerophilic (growing in 5% oxygen) Curved (comma- or S-shaped) Oxidase & Catalase positive Gram-negative rods Nonfermenting Seventy-two hour culture of H pylori showing typical thin, comma- or S-shaped forms Virulence factors Urease Cytotoxin Protease Flagella Pathogenesis H. pylori is associated with type B gastritis (antral stomach inflammation/ peptic ulcer). It shelters from gastric acid in the gastric mucous layer and probably is able to adhere to gastric epithelial cells. Production of urease and cytotoxin is associated with injury to the gastric epithelium. Epidemiology The prevalence of infection increases with age. The source and mode of transmission are not known. H. pylori is in the mucosa of the stomach of 20% people under 30 years but in 40 – 60 % of 60 years old. Detection methods for H pylori Laboratory diagnosis Using endoscopic biopsy samples where the organism can be detected on histological examination Culture PCR (polymerase chain reaction) A rapid urease test on the sample Skirrow’s medium (containing vancomycin, trimethoprim, polymyxin, and amphotericin B.) Laboratory diagnosis Serological tests for antibodies on blood or saliva 13C or 14C urea breath tests Faecal antigen testing 13C or 14C urea breath tests (CUBT) for Helicobacter pylori detection The Carbon urea breath test (CUBT) The breath tests are performed by asking the patient to swallow carbon-labeled urea which is metabolised by H. pylori’s urease to produce labeled carbon dioxide. Two forms of urea breath tests by using 13C urea or 14C urea is available. This is absorbed into the blood stream and then exhaled in the breath of infected individuals. The rapid urease test Least expensive and can be performed on endoscopic biopsy specimens. The urease produced by the organism converts urea to ammonia resulting in a PH change detected by phenol red. The tests usually give a rapid result but typical sensitivity at 1 hour is 71% which increases to 96% at 6 hours. Serological tests for H. pylori Elisa Complement fixation Latex agglutination Serology Testing IgG is the most sensitive as seen in 95%. Testing IgA responses in 68-80%. Testing IgM responses in only 14% of infected patients. Control A three drug treatment for 2 weeks: 1. A proton pump inhibitor (such as lansoprazole and omeprazole decreasing stomach's production of acid allowing the ulcer to heal) 2. Methronidasol 3. Tetracycline Brucella Portals of entry for Brucella species Spread of Brucella in the body Brucellosis is a disease of mainly cattle, swine, goats, sheep and dogs. The infection is transmitted to humans by animals through direct contact with infected materials like afterbirth or indirectly by ingestion of animal products and by inhalation of airborne agents. Consumption of raw milk and cheese made from raw milk (fresh cheese) is the major source of infection in man. Most of the fresh cheeses are sheep and goat cheese. Next to this it is considered to be an occupational disease for people who work in the livestock sector. Human-to-human transmission is very rare.