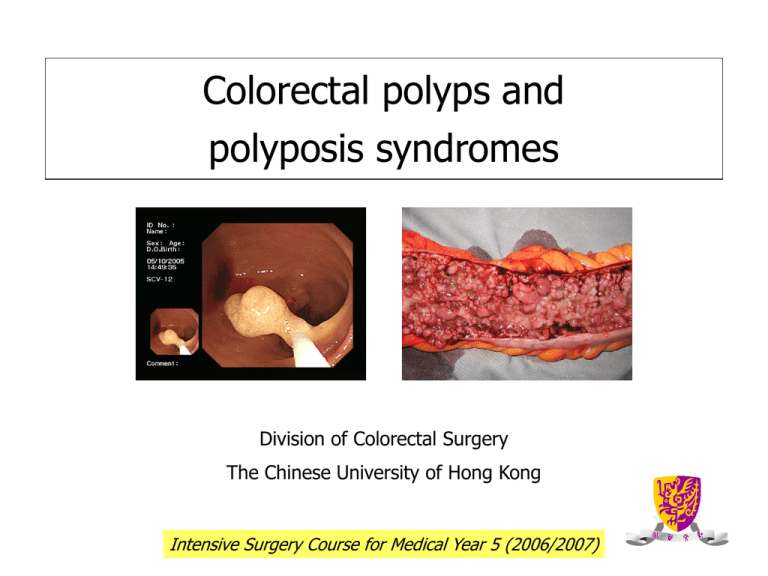
Colorectal polyps and
polyposis syndromes
Division of Colorectal Surgery
The Chinese University of Hong Kong
Intensive Surgery Course for Medical Year 5 (2006/2007)
Colorectal polyps
• visible protrusion above
the surface of the
surrounding normal large
bowel mucosa
• Detected by endoscopy or
by DCBE
Classification of colorectal polyps
Histological classification
Polyp type
Malignant potential
Non-neoplastic
Hyperplastic
No
Hamartomatous
(juvenile, Peutz-Jeghers)
Lymphoid
Inflammatory
Neoplastic (adenoma)
Tubular adenoma
(0-25% villous tissue)
Tubulovillous adenoma
(25-75% villous tissue)
Villous adenoma
(75-100% villous tissue)
Yes
Hyperplastic polyps
• Majority of non-neoplastic polyps
• Prevalence rates of 20-34% (autopsy
and screening colonoscopy studies)
• Predominantly located in the distal
colon and rectum
• Generally small (<0.5cm) in size
Adenomas – facts and figures
•
70% of all colorectal polyps
•
Increase with age (33% of population by 50yr, and in 50% by 70yr)
•
70% located in the left colon
•
70% are solitary (30% synchronous)
•
70% are small (<1cm in size)
•
7% have severe dysplasia, 3-5% have invasive cancer
Adenoma-carcinoma sequence
10 years
Adenoma
CRC
Regardless of aetiology, most CRC arise from adenomas
Factors determining risk of malignant
transformation within adenomas
High risk
Low risk
Large size ( >1.5cm)
Small size ( <1cm)
Sessile or flat
Pedunculated
Severe dysplasia
Mild dysplasia
Villous architecture
Tubular architecture
Polyposis syndrome (multiple polyps)
Single polyp
Percent of adenomas containing
invasive cancer by size and histology
Malignant colorectal polyp
• Polyp that contains invasive cancer
• Malignant cells that have invaded through
the mucularis mucosa into the submucosa
mm
Management of colorectal polyps (1)
Factors
Location: colon or rectum
Morphology: pedunculated or sessile
Histology: benign or malignant
Management of colorectal polyps (2)
Excision
Pedunculated
Colonoscopic polypectomy usually possible
Sessile
Colonoscopic polypectomy if possible (larger polyps may require piecemeal removal)
Endoscopic removable not possible operative removal
• Colon: colectomy
• Rectum: staged with EUS or MRI
• Benign / Early malignant (T1No) : Transanal local excision or TEMS (may need
further radical surgery)
• Other malignant : radical excision (APR /anterior resection)
Management of colorectal polyps (3)
Definitive Mx (histology)
Benign
Malignant
Depends on histological
characteristics
Surveillance
colonoscopy
Radical Surgery
Surveillance after polypectomy
Benign polyps
Characteristics of polyps
Next FU
colonoscopy
small rectal hyperplasic polyps (=average risk)
10 years
one or two small (<1cm) tubular adenomas
5-10 years
3 to 10 adenomas, or adenoma ≥ 1cm, or villous features, or highgrade dysplasia
3 years
>10 adenomas
<3 years
sessile adenomas removed piecemeal
2-6 months
Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the
US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society (2006)
Malignant Polyp
Factors determining need of radical surgery
Histology
• Poorly differentiated
• Margin <2mm
• Stalk invasion
• Lymphovascular invasion
Increase risk of recurrence and LN 2o
Familial Colorectal Cancer Syndromes
Familial adenomatous polyposis (FAP)
• 1% of all CRC
• Present in about 1 in
8000 births
• Autosomal dominant
with near 100%
penetrance
FAP
• >100 adenomas
• Patients develop adenomas by
the mean age of 16 years, and
CRC by 39 years
• Adenomas form early, but it
takes 20-30 years to develop
CRC from adenomas
• Disease of abnormal tumour
initiation
Molecular genetics of FAP
• Caused by mutations of APC gene (tumour suppressor gene) on
chromosome 5q21
• Encodes for a protein, which functions in cell adhesion and signal
transduction
• Mutations will result in truncated protein and affect cell growth
APC as gatekeeper gene
adenoma-carcinoma sequence
Loeb 1991
Mechanisms of Carcinogenesis in
FAP
Genotype vs. phenotype
Affected part of gene
Clinical
Presentation
Extracolonic
manifestations
Cell adhesion and structural
molecules
Extracolonic manifestations
• Congenital hypertrophy of retinal
pigmented epithelium (CHRPE)
• Osteomas, desmoid tumours,
epidermoid cysts (Gardner’s syndrome)
• CNS malignancies including
medulloblastoma and glioblastoma
(Turcot’s syndrome)
• Duodenal, hepatobiliary-pancreatic,
thyroid tumours
CHRPE
Gardner’s syndrome
Desmoid
Chest fibroma
Mandibular osteoma
Skull osteoma
Attenuated FAP (AFAP)
• Variant of FAP
• <100 adenomas
• Late age-of-onset
(adenomas at 44; CRC at 56)
• Proximal distribution of adenomas
*Colonoscopy for surveillance
*Infrequent involvement of the rectum supports the role
of total colectomy and IRA
Cancer risks in FAP
Cancer
Cancer risks
Colon
Near 100%
Duodenal or periampullary
5-10%
Pancreatic
About 2%
Thyroid
About 2%
Gastric
About 0.5%
CNS, usually cerebellar
medulloblastoma (Turcot's syndrome)
<1%
Hepatoblastoma
1.6% of children <5 years of age
Diagnosis of FAP
Endoscopy
Genetic tests
Mutation
Protein truncation test
DNA sequencing
Screening of FAP
• Genetic screening of family members for APC mutations
• Annual flexible sigmoidoscopy beginning at age 10-12
until age 40, then every 3-5 years
*If polyposis is present, colectomy should be considered
• OGD every 1-3 years is also recommended to evaluate
for upper GI adenomas
Prophylactic colectomy for FAP
FAP
CRC
• The aim of surgical treatment of FAP is to intervene in
the adenoma-carcinoma sequence by removing the
adenomas before the transformation to malignancy
occurs
Timing of surgery
Clinical presentation
Asymptomatic patient with modest
number of small adenomas
Timing of surgery
Able to wait for a few years for surgery,
as long as colonoscopic surveillance is
performed yearly
Symptomatic patient with large
number of adenomas
Early surgery
Suspicious of CRC
Very early/urgent surgery
Standard surgical treatment
Restorative proctocolectomy with ileal pouch-anal anastomosis
Suitable for most patients with FAP
Other surgical options
Total colectomy with ileorectal
anastomosis (IRA)
Proctocolectomy with ileostomy
low rectal cancers
Attenuated FAP
poor sphincters
Desmoid tumors
Medical treatment of FAP?
• Sulindac (NSAID) and celecoxib (COX-2 inhibitor) shown to
control and reduce the number of colorectal adenomas in FAP
• Not definitive treatment
• Temporizing treatment (eg when surgery needs to be delayed)
• May control pouch and rectal polyposis after initial prophylactic
surgery
Hereditary nonpolyposis
colorectal cancer (HNPCC)
Dr. A. S. Warthin and the first
HNPCC pedigree, ‘the family G’
1895
Dr. Henry Lynch first described the term
‘cancer family syndrome’ in 1966 (later
renamed as Lynch syndrome and HNPCC)
HNPCC
• 2-5% of all CRC
• Autosomal dominant
• 70-80% penetrance
• It takes only 3-5 years to
develop CRC from adenomas
Accelerated progression
HNPCC: Lynch syndromes
Lynch syndrome I
Early onset of CRC (40-45 years)
Predominantly proximal to the splenic
flexure (60-70%)
Increase frequency of synchronous and
metachronous lesions (33%)
Lynch syndrome II
Features of Lynch Syndrome I +
extracolonic malignancies
*Gastric, small bowel, hepatobiliary,
endometrial, ovarian, ureteral and renal
tumours
HNPCC related extracolonic
tumors
100%
80%
78%
60%
43%
40%
19%
20%
0%
Colorectal
Endometrial
Stomach
18%
Biliary tract
10%
9%
Urinary tract
Ovarian
Endometrial cancer is the most common extracolonic malignancy
Diagnosis: Amsterdam criteria 1
Due to lack of phenotypic markers like polyps
Diagnosis is based on family history of CRC only
1. One member less than 50 years of age
2. Two involved generations
3. Three family members affected, one of whom is a
first-degree relative of the other two
Diagnosis: Amsterdam criteria 2
Same as Amsterdam 1 but
includes all HNPCC
related tumors
Molecular genetics of HNPCC
HNPCC is caused by mutations of
DNA mismatch repair (MMR) genes
Survey DNA for
replication errors
Molecular genetics of HNPCC
• Mutations of these MMR genes will result in replication errors during
DNA synthesis (microsatellite instability) leading to acceleration of
genetic mutations
• HNPCC patients develop adenomas at the same rate as the general
population
• Once these adenomas develop, however, defective DNA repair
ensues and mismatches accumulates
• Thus, it takes only 3-5 years to develop CRC from adenomas
Molecular genetics of HNPCC
Demonstration of MSI
DNA Normal Tissue
DNA Tumor Tissue
Microsatellite Markers
Amplify by Polymerase Chain Reaction
Compare normal and tumor profiles of
amplified microsatellite on gel to detect
genetic mutations in these microsatellites
MSS Tumor
BAT 26
D5S346
NORMAL
MUCOSA
TUMOR
BAT 25 D17S250
D2S123
MSI Tumor
NORMAL
TUMOR
HNPCC:
Mutation detection for MLH1 and MSH2
Microsatellite
instability testing
Negative:
Stop?
Positive
Immunohistochemistry
MLH1
Sequence MLH1
No protein
Sequence MSH2
No protein
MSH2
Normal
Stop?
Screening of HNPCC
• Colonoscopy every 2 years starting at ages 20-25
or
5 years younger than the earliest diagnosis of CRC
whichever is earlier until 40yr , and then annually
• Flexible sigmoidoscopy is not acceptable, due to the proximal
location of tumours
• Transvaginal US and endometrial aspiration annually starting at ages
25-35 years are also recommended
Surgical treatment of HNPCC
• Total colectomy with ileorectal anastomosis
• Restorative proctocolectomy with ileal pouch-anal anastomosis
• Segmental colectomy not recommended because of high rate
of metachronous CRC
• TAHBSO for endometrial cancer
Hamartomatous polyposis syndromes
Peutz-Jeghers syndrome
• Incidence: 1 in 200,000 persons
Autosomal dominant
• Mutations of the STK11 gene on
chromosome 19
• Characterized by perioral
pigmentations and hamartomatous
polyps throughout the GI tract
• GI and non-GI cancers are common
Site of polyps
Frequency
Stomach
38%
Small bowel
78%
Colon
42%
Rectum
28%
Cancer risk in P-J syndrome
GI cancers
Cancer risks
Colon
39%
Pancreatic
36%
Stomach
29%
Small bowel
13%
Esophagus
0.5%
Non-GI cancers
Cancer risks
Breast
54%
Ovarian
21%
Uterine
9%
Sex cord tumour with annular tubules (SCTAT)
20% become malignant
Sertoli cell tumour
10-20% become malignant
Lung
15%
Juvenile polyposis
•
Presence of 10 juvenile polyps in the
GI tract
•
Incidence: 1 in 100,000 persons
•
Autosomal dominant
•
Mutation of SMAD4 gene on
chromosome 18
•
Polyps are most commonly found in
colon
•
Colon cancer risk 50%
•
Risk of gastric, duodenal, and
pancreatic cancers