Bacillus anthracis
advertisement

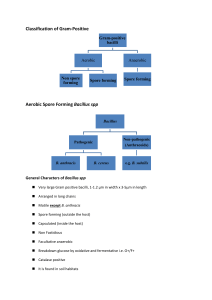
Bacterial agents of bioterroism Laboratory network for biological terrorism Bacillus anthracis Anthrax Anthrax: Overview • Primarily disease of herbivores • Humans usually infected by contact with infected animals or contaminated animal products • Soil reservoir • Woolsorter’s disease (inhalation anthrax) • No person-to-person transmission of inhalational anthrax CDC ANTHRAX • Three forms of human anthrax occur: 1. Cutaneous 2. Gastrointestinal • Oropharyngeal • Abdominal 3. Inhalation (Woolsorter’s Disease) Cutaneous anthrax Vesicle development, day 2 Eschar formation, day 4 Inhalation Anthrax • Infective dose = 8,000 - 15,000 spores • Incubation period = 1-6 days • Duration of illness = 3-5 days • Fever, malaise, and fatigue • Short period of improvement = up to 2 days • Abrupt respiratory distress…death <24hrs • Person to person transmission = no Anthrax: Specimen Selection • Inhalation: Sputum and Blood • Cutaneous: Vesicles and Eschar • Gastrointestinal: Stool and Blood Bacillus anthracis Key Sentinel Lab Tests • • • • • Gram stain Growth characteristics on agar Sporulation, in air Motility Capsule by India Ink Bacillus anthracis Gram Stain Morphology • Broad gram-positive rod: 1-1.5 X 3-5 µ • Oval, central - subterminal spores: 1 X 1.5 µ with no significant swelling of cell • Spores are NOT usually present in clinical specimens unless exposed to atmospheric O2 B. anthracis, Gram stain demonstrating spores B. anthracis Colonial Morphology • Colonial morphology of 18-24hr @ 35 C: – Well isolated colonies are 2-5 mm in diameter – Flat or slightly convex, irregularly round – Edges: slightly undulate, often curly tailing edges – Ground glass appearance – “Sticky” consistency….stands up like beaten egg whites B. anthracis, colony on SBA “STICKY” consistency of B. anthracis’ colony on SBA Bacillus anthracis Presumptive Identification • Gram-positive, broad rod, catalasepositive, spore-positive, aerobe: Bacillus sp. • Spores are oval and nonswelling with ground glass colony appearance: Bacillus morphology group 1, includes B. anthracis, B. cereus, B cereus var mycoides, and B. thuringiensis Bacillus anthracis Presumptive Identification, con’t • Nonmotile: B anthracis and B cereus var mycoides (and B. megaterium) • Nonhemolytic, forms capsule: Presumptive B. anthracis • Refer to state lab for testing Yersinia pestis Plague Plague: Overview • Natural vector - Rodent flea • Mammalian hosts – rats, squirrels, chipmunks, rabbits, and carnivores • Enzootic or Epizootic Plague Epidemiology • U.S. averages 13 cases/yr • 30% of cases are in Native Americans in the Southwest. 15% case fatality rate • Most cases occur in summer and near the patient’s residence – bubonic (infected lymph nodes) – septicemic (blood-borne organisms) – pneumonic (transmissible by aerosol; deadliest) Yersinia pestis Specimen Selection • Specimen selection is important – Bubo - lymph node aspirate – Blood - organisms may be intermittent. Take three specimens 10-30 minutes apart – Pneumonic • Sputum/throat - use Wayson stain • Bronchial washings - Wayson stain • Inoculate routine plating media Sentinel Lab Procedures Yersinia pestis • Gram stain • Wayson stain • Growth characteristics on agar • Growth characteristics in broth Yersinia pestis Gram stain • Small, gram-negative coccobacilli Yersinia pestis Wayson Stain • Used for rapid assessment – when it is a part of the identification process • Best with tissue, sputum, blood • Stains of pure culture isolates tend to lose bipolarity • Pink-blue cells with polar granules (safety pin appearance) Yersinia pestis Wayson Stain • Pink-blue cells with a closed safety pin look Wayson stain alone is not diagnostic Y.pestis 48 h culture on SBA 72 h culture on SBA Yersinia pestis Technical Hints • Small Gram-negative, poorly staining rods from blood, lymph node aspirate, or respiratory specimens • Safety pin appearance in Gram, Wright, Giemsa, or Wayson stain • More than one patient in a short, specified period with fever, lymphadenopathy • Refer to state lab Francisella tularensis Tularemia Tularemia: Overview • Disease of Northern Hemisphere • In U.S., most cases associated with rabbits/hares and ticks • About 200 cases/year in U.S. – most in South central and Western states – majority of cases in summer, some in winter Reported Cases of Tularemia 1990-1998 Tularemia: Overview (cont’d) • Several forms of human tularemia exist: - Ulceroglandular, glandular, oculoglandular, oropharyngeal, intestinal, pneumonic, and typhoidal • Low infectious dose –1 to 10 organisms by aerosol or intradermal route • No person-to-person transmission Tularemia: Specimen Selection • Serum - acute and convalescent • Blood cultures • Sputum • Swab – ulcer or eye Sentinel Lab Procedures Francisella tularensis • This is a dangerous, highly virulent organism and it should not be manipulated at the bench. Laboratory-acquired infections can occur easily. • Gram stain • Growth characteristics in broth • Growth characteristics in agar Francisella tularensis • Poorly staining, tiny Gram-negative coccobacilli Francisella tularensis Growth Characteristics • Fastidious, requires cysteine for robust growth: Cysteine Heart Agar (CHA) is ideal – Enriched chocolate agar + 9% sheep blood + cysteine – Not part of Sentinel Lab routine procedures – BCYE (for Legionella) also works • Will grow initially on sheep and chocolate blood agar and Thayer-Martin agar, but poorly or not at all on passage • Grows slowly at 35oC, poorly at 28oC Francisella tularensis Growth Characteristics • 24 hours – gray-white, translucent colonies – usually too small to be seen individually • 48 hours – Sheep Blood Agar - <1 mm, gray-white, opaque, no hemolysis Francisella tularensis Sheep blood agar Chocolate agar Cysteine heart agar Francisella tularensis Technical Hints If you see: • Tiny, Gram-negative coccobacilli from blood, lymph node aspirate, or respiratory specimens • Blood isolates that will grow slowly on chocolate agar but poorly or not at all on blood agar in 24 hours • Faint growth in thio; requires cysteine in other broth • Refer to state lab Brucella spp. Brucellosis BRUCELLOSIS • A zoonotic disease caused by any of 4 Brucella sp.: abortus, melitensis, suis, and canis • A systemic infection characterized by an undulant fever pattern • But relatively rare in the U.S. with approximately 100 cases/yr BRUCELLOSIS: TRANSMISSION • Unpasteurized dairy products – The most common mode of transmission • Direct skin contact – Occupational hazard for farmers, butchers, veterinarians, and laboratory personnel • Aerosols – Highly infectious BRUCELLOSIS •Infective dose = 10 -100 organisms •Incubation period = 5 days - > 6 months •Duration of illness = weeks to months •Fever, profuse sweating, malaise, headache and muscle/back pain. •Person to person transmission = no •Mortality = <5% •Persistence of organism = very stable Brucella spp. Specimen Selection • Serum – The diagnosis of brucellosis is frequently achieved by serology. An acute & convalescent phase specimen should be collected (21d apart) • Blood or bone marrow – Sources from which Brucellae are most often isolated • Tissue (spleen, liver) – Brucellae occasionally isolated Brucella spp. Biosafety Alert • Brucellosis is THE most commonly reported laboratory-associated bacterial infection. • Cases have occurred in clinical laboratory settings by “sniffing” cultures, direct skin contact with cultures, and aerosol generating procedures Sentinel Lab Tests Brucella spp. • Colonial morphology on SBA • Gram stain morphology • Oxidase positive • Urea hydrolysis positive Brucella spp. Key Sentinel Lab Tests Colonial morphology on SBA –Fastidious –Visible growth may take 48 - 72 hrs –Small (0.5-1.0mm), convex, glistening – Non-hemolytic and non-pigmented B. melitensis on sheep blood agar Brucella spp. Key Sentinel Lab Tests Gram Stain Morphology –Tiny (very) –Faintly staining –Gram-negative coccobacilli –0.5 - 0.7 x 0.6 - 1.5 Brucella spp. Review of Key Tests • Tiny, faintly staining, gram-negative coccobacilli from blood or bone marrow • Slow growth on Sheep Blood Agar, 2-3 days for colony appearance • Oxidase + • Urease + • Handle plates with care • Refer to state lab Clostridium botulinum Botulism Botulism: Overview • Caused by toxin from Clostridium botulinum – toxin types A, B, E, most commonly associated with human disease – most potent lethal substance known to man (lethal dose 1ng/kg) • C. botulinum spores found in soil worldwide • Approximately 100 reported cases/year in the U.S. – infant most common (72%) – food borne not common • No person-to-person transmission FOODBORNE BOTULISM • Infective dose: 0.001 g/kg • Incubation period: 18 - 36 hr (6hr to 10 d) • Dry mouth, double vision, droopy eyelids, dilated pupils • Generalized, progressive descending bilateral muscle weakness & paralysis • Respiratory failure and death • Mortality usually 5 – 10% FOODBORNE BOTULISM • Among 309 persons with clinically diagnosed botulism reported to CDC from 1975 to 1988: – Stool cultures for C. botulinum: 51% + – Serum botulinum toxin testing: 37% + – Stool botulinum toxin testing: 23% + • Overall, at least one of the above tests was positive for 65% of all patients • Diagnosis is primarily clinical Sentinel Lab Procedures for Botulism Event • Properly collected specimens are to be referred to designated testing laboratories • Prior to the shipment of any botulismassociated specimen, testing must be arranged with MDCH laboratory Sentinel Lab Procedures for Botulism Event Clinical specimens to be collected: 1. Serum 2. Feces 3. Food samples Autopsy specimens: 1. Serum 2. Gastric and intestinal contents Botulism Biosafety Alert Materials suspected of containing botulism toxin must be handled: –Biological Safety Cabinet (Class II) –Laboratory Coats –Disposable surgical gloves –Face shield (as needed) BOTULISM • The diagnosis of botulism is made clinically, i.e., based on the patient’s case history and physical findings • Health care providers suspecting botulism should contact the Michigan Department of Community Health Botulism Referral Lab Procedures • Mouse bioassay • Isolation of C. botulinum