ICAAC-2010-uti-and-thigh
advertisement

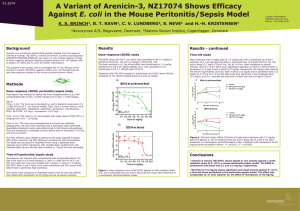
F1-2075 Activity of Arenicin-3 Variant, NZ17074, Against E. coli in the Mouse Urinary Tract and Thigh Infection Models K. S. BRINCH1, B. T. RAVN1, C. V. LUNDBERG2, S. NEVE1 and H.-H. KRISTENSEN1 1Novozymes Background Arenicin-3 is a ß-hairpin antimicrobial peptide isolated from the lugworm (Arenicola marina). NZ17074, a variant of arenicin-3 (amino acid sequence: GFCWNVCVYRNGVRVCHRRCN), has shown in vitro activity against a range of Gram-negative bacteria including resistant strains of E. coli (please refer to posters F1-2070 and F1-2071 for further information). The purpose of these studies was to investigate the in vivo efficacy of arenicin variant NZ17074 against two multi-resistant strains of E. coli in the neutropenic thigh infection and the mouse urinary tract infection (UTI) model. Methods Neutropenic thigh infection study Neutropenia was induced by dosing the mice intraperitoneally (i.p.) with cyclophosphamide on Day -4 (200 mg/kg) and on Day -1 (100 mg/kg). Day 0: Time = -1 hrs: The mice were inoculated in one thigh with 50 uL of a bacterial suspension of 2 x 10E7 CFU/ml of E. coli (clinical isolate, 2003, from a human wound; multiresistant (ampicillin, ceftazidime, aztreonam, gentamicin, ciprofloxacin). MICs: NZ17074: 0.5 mg/L; meropenem: 0.25 mg/L. Time = 0 hrs: The mice (n=4) were treated i.v. with single doses of NZ17074 ranging from 0.18 – 12 mg/kg. Time = 5 hrs: The mice were euthanized and quantitative bacterial counts were determined in the thighs and compared to untreated controls before start of treatment (T=0 hrs) and five hours later. Treatment with meropenem at 40 mg/kg served as positive control. A/S, Bagsvaerd, Denmark, 2Statens Serum Institut, Copenhagen, Denmark Results Results – continued Neutropenic thigh infection study Also in the bladder and kidneys, a significant reduction of 2.6 and 1.6 log10 CFU/ml, respectively, was seen after i.v. treatment with 10 mg/kg NZ17074 (p<0.01 and p<0.05). The ED50 values for the E. coli strain AID#172 was calculated to 5.9 mg/kg.(Fig. 1). The dose resulting in a 1 log killing effect was determined to 6.1 mg/kg and Emax was calculated to 2.4 log10 CFU/ml. Treatment with NZ17074 resulted in significantly lower bacterial counts compared to the vehicle-treated group at 6 (p<0.05) and 12 mg/kg (p<0.01). The effect on meropenem at 40 mg/kg did not reach a statistically significant level (data not shown). Treatment with meropenem at 40 mg/kg resulted in a significant reduction of 1.4 log10 CFU/ml in the bladder (p<0.01). In contrast, the effect of meropenem did not reach a significant level in the kidneys where treatment resulted in a reduction in bacterial load of 0.8 log10 CFU/ml (Fig. 3). Figure 1. Dose-response curve of activity of NZ17074 against a multiresistant strain of E. coli in thighs five hours after treatment in a neutropenic thigh infection model. Urinary tract infection study Treatment with NZ17074 at 10 mg/kg reduced the bacterial loads in the urine with 3.2 log10 CFU compared to the initial bacterial load. In vehicle-treated mice the CFU levels in the urine remained the same during the study period. After treatment was completed on Day 3 mice treated with NZ17074 had a significantly lower CFU level than the vehicle group (p<0.001). Treatment with meropenem at 40 mg/kg resulted in a significant reduction of CFU/ml (4 log10, p<0.001) (Fig. 2). The 50% effective dose (ED50) is defined as the dose required to obtain 50% of the maximum log CFU difference compared to start of treatment. The ED50 was calculated in GraphPad Prism using a sigmoidal dose-response curve (Hill’s regression) with variable slope. Furthermore, the maximal effect (Emax) and the dose resulting in 1 log10 kill were calculated. Figure 3. Bacterial counts (log10 CFU/ml) in the bladder and kidneys on Day 3 after infection with E. coli AID#172 and twice daily treatment with NZ17074 at 10 mg/kg or meropenem at 40 mg/kg i.v. ns: not significant; *: p<0.05; **: p<0.001. Conclusions Urinary tract infection (UTI) study • Arenicin-3 variant, NZ17074, shows in vivo activity against a multi-resistant strain of E. coli in the neutropenic thigh infection model. The ED50 in the thigh muscle was calculated to 5.9 mg/kg, the 1 log killing dose was 6.1 mg/kg and the Emax was 2.4 log10 CFU/ml. Starting Day -4 before inoculation mice were given drinking water with 5% glucose. On Day 0 mice were anaesthetized and inoculated in the bladder with 50 uL bacterial suspension (1x109 CFU/ml) of E. coli (clinical isolate, 2003, from a human wound; multiresistant (ampicillin, ceftazidime, aztreonam, gentamicin, ciprofloxacin). On Day 1 and 2 the mice (n=6-7) were treated twice daily with 10 mg/kg NZ17074 i.v. Urine samples were taken on Day 1 before the first treatment and on Day 3 the mice were sacrificed and urine, kidneys and bladder were collected for CFU counts. The bacterial counts were compared to untreated controls and mice treated with meropenem at 40 mg/kg by one-way ANOVA. Contact information: KBri@novozymes.com Phone: +45 4446 4787 Figure 2. Bacterial load in the urine before treatment (Day 1) and after last treatment (Day 3) with NZ17074 or meropenem after infection with E. coli. ***: p<0.001 • NZ17074 also shows potent activity in the urinary tract infection model against a multi-resistant strain of E. coli. Two daily dosings with 10 mg/kg significantly reduced the bacterial count in both urine, bladder and kidneys at levels comparable to meropenem dosed at 40 mg/kg. • NZ17074, and optimized variants hereof, are promising drug candidates against infections with Gram-negative bacteria.