(PK) and Efficacy of TP-433 in Murine Infection Models Challenged
advertisement

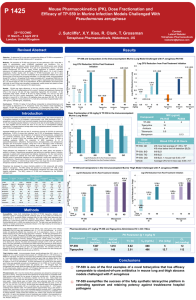
P 1426 The Intravenous Pharmacokinetics (PK) and Efficacy of TP-433 in Murine Infection Models Challenged with Pseudomonas aeruginosa Y. Deng, T. Grossman, R. Clark, X.-Y. Xiao, J. Sutcliffe* Tetraphase Pharmaceuticals, Watertown, US Abstract T1/2 (hr) 2.55 C0 (ng/mL) 487 10 6 4 cfu reduction: m g/ kg IV g/ kg 40 m 40 cl in e ec y tig to br am g/ kg m 40 3 43 TP - yc in IV IV * g/ kg TP -4 3 TP -4 3 3 5 re a un t 3 PA1145 MIC (µg/ml) 4 16 4 0.5 Compound TP-433 Tigecycline Amikacin Tobramycin P. aeruginosa Immunocompetent Murine Thigh Model Log10 CFU per gram thigh @ 24 hr 10 8 6 4 cfu reduction: Time Kill Assays , p0.01 vs. untreated 0 hr control , p0.01 vs. untreated 24 hr control 40 m TP -4 33 33 -4 TP m er g/ op kg en IV em m 5 er m op g/ kg en em IV 15 m er m op g/ kg en em IV 40 m g/ kg IV g/ kg 15 m 5 33 -4 TP IV IV m g/ kg te d re a re a te d 24 0 hr hr 2 un t Pharmacokinetics. CD-1 mice (n=24) were received from Charles River Laboratories and were allowed to acclimate for two days prior to dosing. A single dose was administered to each mouse by intravenous injection via the tail vein. Body weight was measured prior to dosing and mice were dosed at a dose volume of 5 mL/kg based on individual body weight. Study animals were not fasted. Mice (n=3 per time point) were euthanized using CO2 and blood samples were collected at sacrifice by cardiac puncture at eight PK time points (2 min, 30 min, 1 hr, 2 hr, 4 hr, 6 hr, 12 hr, 24 hr). All time points were collected within 5% of the target time. Blood collection (approximately 250 µL at sacrifice) was added into pre-chilled (0 – 4C) heparincontaining blood collection tubes. The collected plasma (approximately 100 µL) was placed in sample tubes, snap frozen on dry ice and immediately stored at nominally -80C. Frozen plasma samples and dosing solution retains were analyzed for TP-433 concentration by LC/MS/MS using an internal standard. Quality control (QC) samples (low, medium, high; minimum of 6 standards with LLOQ < 3 ng/mL) and standard curves (in duplicate) were included in the bioanalytical run. WinNonLin was used to determine individual and mean PK parameters ( Cmax, Tmax, T½, CL, Vss, AUC(0-t), AUC(0-), and MRT). 15 m te d m g/ kg 24 IV * hr * hr 0 te d re a un t Dose Group un t Immunocompetent thigh infection model. Groups of 5 female specific-pathogen-free CD-1 mice weighing 22 ± 2 g were used. On Day 0, animals were inoculated intramuscularly (0.1 ml/thigh) with 3-5 x 106 CFU/mouse of P. aeruginosa PA694, a recent clinical isolate, into the right thigh. Two groups did not receive drug treatment and thighs were harvested at 2 hour post-infection. Remaining mice were administered either vehicle, TP-433, or meropenem at 2 and 12 hours post infection. The muscle of the right thigh of each animal was harvested at 24 hr post-infection. Harvested thigh tissues were homogenized in 2 ml of PBS, pH 7.4 with a Polytron tissue homogenizer. The homogenates were serially diluted and plated on Brain Heart Infusion agar + 0.5% charcoal (w/v) for CFU determination per gram of thigh. Individual animal CFU/gram thigh data was plotted using GraphPad Prism. Mean and standard deviations are show per dose group and statistical significance of dose group vs. T=0 hr or T=24 hr controls was determined by non-parametric Mann-Whitney analysis using GraphPad Prism.. IN , p0.01 vs. untreated 0 hr control , p0.01 vs. untreated 24 hr control *n=5 mice; all other groups n=6 mice Tetraphase’s chemistry platform has enabled the optimization of fully synthetic, novel tetracycline-class molecules with improved activity against P. aeruginosa in vitro (see Poster 1448) and demonstrated efficacy in vivo (this poster and Poster 1425), aimed at addressing this growing medical need. Immunocompetent lung infection model. Female BALB/c mice weighing 18 to 20 grams were infected with ~2 x 107 CFU/mouse of P. aeruginosa PA1145, a cystic fibrosis isolate from Children’s Hospital, Boston, via intranasal administration of 0.05 ml of cell suspension under light anesthesia. One group did not receive drug treatment and lungs were harvested at 2 hour post-infection. At 2 and 12 hours post-infection mice were treated with TP-433, tigecycline or amikacin intravenously, or tobramycin intranasally. Mice (n=6 per group) were treated with each drug concentration. Twenty-four hours post initiation of treatment, mice were euthanized by CO2 inhalation. The lungs of the mice were aseptically removed, weighed, homogenized, serially diluted, and plated on MacConkey medium. The plates were incubated overnight at 37C in 5% CO2. CFU per gram of lung was calculated by enumerating the plated colonies then adjusting for serial dilutions and the weight of the lung. Individual animal CFU/gram lung data was plotted using GraphPad Prism. Mean and standard deviations are show per dose group and statistical significance of dose group vs. T=0 hr (2 hr post-challenge) or T=24 hr (24 hrs post-challenge) controls was determined by non-parametric MannWhitney analysis using GraphPad Prism. Intrinsic resistance due to low permeability of the outer membrane and multidrug efflux pumps, the spread of acquired resistance mechanisms including drug-inactivating enzymes, and the dissemination of isolates with target-based drug resistance mutations has greatly reduced the effectiveness of currently marketed antibiotics against P. aerguinosa infections. MIC assays were performed according to methods published by Clinical and Laboratory Standards Institute (CLSI; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Eighth Edition, 2009. CLSI document M07-A8). MRT (hr) 2.37 8 In rare circumstances P. aeruginosa causes infections such as burn wounds, folliculitis, skin and soft tissue infections, and external otitis in otherwise healthy individuals. Methods CL (mL/min/kg) 47 P. aeruginosa Immunocompetent Murine Lung Infection Model 2 P. aeruginosa is an opportunistic pathogen causing difficult-to-treat infections in compromised individuals such as those afflicted with diabetes, cystic fibrosis, and hospitalized patients who are intubated and undergoing mechanical ventilation. Vss (L/kg) 6.6 40 Introduction AUCinf (ng·h/mL) 357 in Conclusions: TP-433 was efficacious in both P. aeruginosa infection models. In the lung model TP433 was equipotent to amikacin at 40 mg/kg, and more potent than tigecycline at all doses. TP-433 protected less well than meropenem in a neutropenic thigh model. The AUC of TP-433 in mice was 2.6-fold higher than that observed with 1 mg/kg IV tigecycline. AUC0-t (ng·h/mL) 348 IV * Results: TP-433 was efficacious in the mouse lung infection model, providing a 1, 2, or 3-log reduction in CFUs at 3, 12 and 29 mg/kg, respectively, relative to 24-hr controls. Amikacin, a positive control, reduced the CFUs in the lung by 3.55 log CFUs while tigecycline reduced CFUs less than one log. In the neutropenic thigh model, TP-433 at 40 mg/kg provided a 2.2 log reduction from the 24-hr control counts while meropenem reduced bacterial burden ≥4 log CFUs at doses ≥15 mg/kg. The PK of 1 mg/kg IV in mice produced a t½, AUC(0-t), and Cmax of approximately 3 hr, 348 ng·h/mL, and 487 ng/mL, respectively. PK Parameter @ 1 mg/kg IV g/ kg Methods: Lung infection model: Immunocompetent female BALB/c mice (18-20 grams) were infected with cystic fibrosis isolate P. aeruginosa PA1145 (TP-433 MIC = 4 mcg/ml) via intranasal (IN) administration. At 2 and 12 hours (hr) post-infection, mice (n = 6) were treated intravenously (IV) with TP-433 (5, 15, or 40 mg/kg), tigecycline (40 mg/kg), or amikacin (40 mg/kg). Mice were euthanized by CO2 inhalation 24 hr post-initiation of treatment. Lungs were removed, weighed, homogenized, serially diluted, plated on MacConkey agar, and colony forming units (CFUs) per gram of lung were calculated. Thigh model: Groups of 5 female CD-1 mice (18-20 grams) were made neutropenic by administration of cyclophosphamide on Days -4 and -1. On Day 0, mice were inoculated with P. aeruginosa PA694 (TP-433 MIC = 4 mcg/ml) into the right thigh. TP-433, tigecycline, and meropenem were administered at 5, 15, and 40 mg/kg IV 2 and 12 hr post-infection. At 24 hr post-infection, the muscle of the right thigh of each mouse was harvested, homogenized, serially diluted and plated on Brain Heart Infusion agar + 0.5% charcoal for CFU determination. PK evaluation (WinNonLin) of TP-433 and tigecycline were performed in CD-1 mice after 1 mg/kg IV administration and collection of 8 plasma samples over 24 hr. TP-433 levels were quantified by LC/MS/MS. Pharmacokinetics of TP-433 administered IV to BALB/c mice Log10 CFU per gram lung @ 24 hr Objective: To determine the PK in mice and to examine the efficacy of TP-433 in mouse lung and infection models challenged with P. aeruginosa. Results m ECCMID 31 March – 3 April 2012 London, United Kingdom Contact: Leland Webster Tetraphase Pharmaceuticals lwebster@tphase.com am ik ac 22nd Dose Group Compound TP-433 Meropenem PA694 MIC (µg/ml) 4 0.5 Conclusion TP-433 is a novel anti-Pseudomonas tetracycline that shows good pharmacokinetic properties in mice and strong efficacy in P. aeruginosa lung and thigh infections

![Historical_politcal_background_(intro)[1]](http://s2.studylib.net/store/data/005222460_1-479b8dcb7799e13bea2e28f4fa4bf82a-300x300.png)