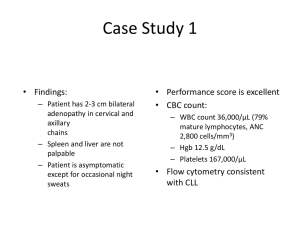
- Oncology Exchange
advertisement

Faculty Disclosures The faculty reported the following relevant financial relationships that they or their spouse/partner have with commercial interests: • Presenter, MD: Research: Pharma Company; Consultant: Pharma Company TO BE FILLED IN BY PRESENTING PHYSICIAN(S) Off-label discussion disclosure: This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the Food and Drug Administration. PCME does not recommend the use of any agent outside of the labeled indications. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings. The opinions expressed are those of the presenters and are not to be construed as those of the publisher or grantors. Steering Committee Disclosures The Steering Committee reported the following relevant financial relationships that they or their spouse/partner have with commercial interests: • John P. Leonard, MD: Advisor/Consultant: Abbott, Biotest, Calistoga Pharmaceuticals/Gilead, Celgene, Cell Therapeutics, Cephalon, GlaxoSmithKline, Hospira, Immunomedics, Johnson & Johnson, Medimmune, Millennium Pharmaceuticals, Morphosys, Sanofi Aventis, Seattle Genetics • Julie Vose, MD, MBA: Research Grant: Allos Therapeutics, BristolMyers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte Corp., Millennium Pharmaceuticals, Onyx Pharmaceuticals, Pharmacyclics, S*Bio Pte. Ltd, Sanofi-Aventis, US Biotest Non-faculty Disclosures Non-faculty content contributors and/or reviewers reported the following relevant financial relationships that they or their spouse/partner have with commercial interests: Latha Shivakumar, PhD; Bradley Pine; Blair St. Amand; Jay Katz; Paul M. Barr, MD: Nothing to Disclose Educational Objectives At the conclusion of this activity, participants should be able to demonstrate the ability to: • Review the current approaches for the treatment of relapsed/refractory NHL • Compare the various available treatments and choose the optimal treatment based on patient characteristics and recently presented data from ongoing clinical studies in relapsed/refractory NHL • Identify key investigational agents from ongoing clinical studies in relapsed/refractory NHL Paper Audience Response Questions • During the course of the program Audience Response Questions will be asked to determine the current knowledge of the audience • These questions will not be graded and are for outcomes purposes only • Please record your answers on your evaluation form located in your packet Non-Hodgkin Lymphoma (NHL) • Most common hematological cancer • Seventh-most common cancer in United States • Approximately 65,000 cases of NHL diagnosed in 2010 • More than 20,000 deaths from NHL reported in 2010 • As of 2007, approximately 430,000 people were living with a history of NHL in the United States American Cancer Society (ACS). http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf. Flowers CR, et al. CA Caner J Clin. 2010;60(6):393-408. National Cancer Institute (NCI). http://www.cancer.gov/cancertopics/types/non-hodgkin. SEER Stats Fact Sheet: Non-Hodgkin Lymphoma. http://seer.cancer.gov/statfacts/html/nhl.html. Frequency of NHL Subtypes In Adults Mantle cell (6%) Follicular lymphoma (22%) Peripheral T-cell (6%) Other subtypes with a frequency 2% (9%) Small lymphocytic lymphoma (6%) Composite lymphomas (13%) Marginal zone B-cell lymphoma MALT type (5%) Diffuse large B-cell (31%) MALT = mucosa-associated lymphoid tissue. Armitage JO , Weisenberger DD. J Clin Oncol. 1998;16:2780-2795. Marginal zone B-cell lymphoma nodal type (1%) Lymphoplasmacytic lymphoma (1%) Two Major Lymphoma Clinical Paradigms • Aggressive histologies—diffuse large B cell – Practical objective of treatment: cure – Reasonably good clinical prognostic tools – Most patients treated the same (R-CHOP) – Unmet need: one-third not cured; reduce toxicity of treatment • Indolent histologies – Practical objective of treatment: long disease control – Fair clinical prognostic tools – Treatment choice balances efficacy and toxicity – Unmet needs: cures, rational treatment selection R-CHOP = cyclophosphamide, doxorubicin, vincristine, and prednisolone, with rituximab. Emerging Prognostic Tools for FL • Clinical – IPI, FLIPI, FLIPI-2 • Pathologic • Molecular profiling • Response-based – MRD, CR vs PR, PET – Duration of current/prior response – Interesting potential, but limited data to date, indicating that these should be used to guide therapy or drug development FL = follicular lymphoma.; IPI = International Prognostic Index; FLIPI = Follicular Lymphoma International Prognostic Index; FLIPI-2 = Follicular Lymphoma International Prognostic Index 2; MRD = minimal residual disease; CR = complete response; PR = partial response; PET = positron emission tomography. Overall Survival In FL by FLIPI L Elevated LDH A Age ≥60 S Stage III/IV H Hemoglobin <120 g/L Survival Probability No Nodal regions 4 1.0 Intermediate risk 0.6 High risk 0.4 0.2 0 Risk Group Low Intermediate A High No. of Factors 0-1 2 3-5 LDH = lactate dehydrogenase; OS = overall survival. Adapted from Solal-Celigny et al. Blood. 2004;104:1258. Low risk 0.8 P < 10-4 0 1 2 % of Patients 36 37 27 3 y 4 5 5-y OS (%) 90.6 77.8 52.5 6 7 10-y OS (%) 70.7 50.9 35.5 Current Treatment Options in Indolent Lymphoma • Observation • Single-agent rituximab ± maintenance – Can adding other biologics enhance activity without major toxicity? • Chemotherapy + rituximab ± maintenance – What is the best chemotherapy? – What is the role of maintenance rituximab? • RIT – Alone or as consolidation • SCT options in first (second, later) remission • Novel/investigational agents RIT = radioimmunotherapy; SCT = stem cell transplantation. Managing Patients With Relapsed and Refractory FL Outcomes With Sequential Chemotherapy in FL—1995 • 212 patients, newly diagnosed FL over 20 years • Treated generally with chlorambucil, CVP, CHOP CVP = cyclophosphamide, vincristine, and prednisone. Johnson PWM et al. J Clin Oncol. 1995;13(1):140-147. 1st Line Median Response Duration (months) 31 Survival From Response (years) 9.6 2nd Line 13 4.9 3rd Line 13 3.5 4th Line 6 1.2 Limitations of These Data • Retreatment with same agents versus different approaches • Pre-rituximab era • New therapeutic options now available • More rigorous monitoring/surveillance common now – For better or worse Case 1 • A 60-year-old male presented with follicular, grade 2 NHL with symptomatic diffuse LAN in the 3- to 4-cm range; he is treated with R-CHOP × 6 cycles and then observed. • 2.5 years later, he presents with axillary and abdominal LAN in the 3- to 4-cm range on routine imaging • Blood chemistries, LDH, and complete blood counts are normal, and bone marrow biopsy shows no evidence of involvement with lymphoma LAN = Lymphadenopathy ARS Question 1 Please record your answer on your evaluation form now. The preferred management for this patient is: A. Close observation and monitoring B. Single-agent rituximab × 4 doses C. Single-agent rituximab + maintenance rituximab D. Rituximab-fludarabine combination regimen E. Bendamustine-based regimen F. RIT G. RICE or similar regimen H. RICE or similar regimen, followed by ASCT RICE = rituximab, ifosfamide, carboplatin, etoposide; ASCT = autologous SCT. ARS Question 1 The preferred management for this patient is: A. Close observation and monitoring B. Single-agent rituximab × 4 doses C. Single-agent rituximab + maintenance rituximab D. Rituximab-fludarabine combination regimen E. Bendamustine-based regimen F. RIT G. RICE or similar regimen H. RICE or similar regimen, followed by ASCT All the answers are reasonable choices for this patient. RICE = rituximab, ifosfamide, carboplatin, etoposide; ASCT = autologous SCT. Considerations in Choice of Therapy for Second-line Treatment • • • • • Consider transformation Symptoms Bulk of disease Age and performance status Efficacy of last treatment – Response, time since, tolerability – Similar versus something different • Patient preferences Specific Considerations for This Patient • • • • Transformation clinically unlikely No symptoms Nonbulky disease Age and performance status – SCT option, perhaps • Last treatment – Effective but would prefer more durability Treatment Options for This patient • Chemotherapy + rituximab – Bendamustine favored over fludarabine • Single-agent rituximab – Asymptomatic, but unlikely to be durable • RIT – Well tolerated, chance at durability • Combination chemotherapy + SCT – Potentially durable, though toxicity Rummel MJ et al. Blood 2010;116: Abstract 856. Chemotherapy Data for Recurrent Indolent NHL (Until Recently) Regimen N RR TTF/PFS (mo) Rituximab monotherapy 166 48% 13 FCM 30 70% 21 Rituximab + FCM 35 94% Not reached (median follow-up: 3 y) CHOP 231 72% 20 R-CHOP 234 85% 33 FCM = fludarabine, cyclophosphamide, mitoxantrone; RR = relative risk; TTF = time to treatment failure; PFS = progression-free survival. McLaughlin P et al. J Clin Oncol. 1998;16:2825; Forstpointner R et al. Blood. 2004;104:3064; Van Oers. Blood. 2006;108:3295; Vose. J Clin Oncol. 2000;18:1316; Witzig. J Clin Oncol. 2002;20:2453; Witzig TE et al. J Clin Oncol. 2002;20:3262; Horning. J Clin Oncol. 2005;23:712. Caveats From Data in This Population • Heterogeneous prior therapies • Heterogeneous prior use of rituximab • Heterogeneous prior use of R-CHOP and rituximabbendamustine • How much these data apply to an individual patient today is unclear • Expect that, with better upfront therapy, a “resistant” patient will be “worse” with respect to prognosis Phase II Trial of Bendamustine in Rituximabrefractory NHL: Results Multicenter, nonrandomized, open-label, single-arm trial Patient Subset N ORR CR/CRu PR DOR (mo) Evaluable patients 74 77% 34% 43% 6.67 Patients with ≥2 prior therapies 40 75% 30% 45% 5.3 Prior ASCT 6 67% 17% 50% 2.6 Alkylator-refractory patients 23 61% 13% 48% 7.7 Fludarabine-refractory patients 8 62% FL 45 81% 37% 44% SLL 11 63% 36% 27% LPL 1 100% 100% 0 MZL 2 100% 50% 50% Transformed 15 66% 13% 53% Prior Therapies NR Histology NR Cru = unconfirmed complete response; DOR = duration of response; NR = not reported; ORR = overall response rate; SLL = small lymphocytic lymphoma; LPL = lymphoplasmacytic lymphoma; MZL = marginal zone lymphoma.. Friedberg JW et al. J Clin Oncol. 2008;26:204-210. Bendamustine in Rituximab-refractory NHL: PFS PFS (All Treated Patients) PFS (Indolent vs Transformed) CI = confidence interval. Friedberg JW et al. J Clin Oncol. 2008;26:204-210. Reprinted with permission. © 2008 American Society of Clinical Oncology. Bendamustine + Rituximab in Relapsed/ Refractory Indolent and MCL: Efficacy Efficacy: FL ORR CR PR No. of Patients (%) MCL Marginal Zone SLL (n = 24) (n = 17) 23 (96%) Total (n = 16) (n = 6) (n = 63) 17 (100%) 12 (75%) 5 (83%) 57 (90%) 17 (71%) 9 (53%) 8 (50%) 4 (67%) 38 (60%) 6 (25%) 8 (47%) 4 (25%) 1 (17%) 19 (30%) Median duration of PFS: 30 months Median OS: 65 months Safety: Grade 3 Grade 4 % of Grade 3/4 Leukocytopenia 32 3 16% Thrombocytopenia 6 1 3% Anemia 2 - 1% MCL = mantle cell lymphoma. Robinson KS, et al, J Clin Oncol 2008; 26: 4476-79; Rummel ASCO 2007, Abstract 8034. Radiolabeled Anti-CD20 Antibodies • Yttrium-90 ibritumomab tiuxetan (Zevalin®) • Iodine-131 tositumomab (Bexxar®) • Pretargeted (investigational) – Secondary agent to amplify radiation dose and enhance specificity Common Themes Regarding Radiolabeled Anti-CD20 Antibodies • • • • • • • • Treatment done in a week (2 injections) Gamma camera imaging or counts a component Manageable radiation safety issues Toxicity is principally infusion-related and hematologic – Baseline hematologic status relevant – Blood count monitoring required for 3 months Important long-term toxicity usually absent RR, 60%-80%; CR, 30% Some patients have durable remissions Myeloablative therapy promising ARS Question 2 Please record your answer on your evaluation form now. Where should you consider the use of radioimmunotherapy (RIT) in NHL? A. Relapsed low-grade/transformed NHL B. Chemotherapy-refractory low-grade NHL C. Rituximab-refractory low-grade NHL D. Transformed NHL E. All of the above ARS Question 2 Where should you consider the use of radioimmunotherapy (RIT) in NHL? A. Relapsed low-grade/transformed NHL B. Chemotherapy-refractory low-grade NHL C. Rituximab-refractory low-grade NHL D. Transformed NHL E. All of the above Radioimmunotherapy (RIT) for NHL • Current data clearly support use in: – Relapsed low-grade/transformed NHL Advantages over rituximab + chemotherapy debatable – Chemotherapy-refractory low-grade NHL – Rituximab-refractory low-grade NHL – Transformed NHL – Responsive disease but with short remissions • Potential utility in: – Upfront therapy (alone or in sequence with chemotherapy) – Relapsed/refractory patients with other histologies Remission Duration of Patients Receiving ASCT for FL in Second or Later Remission Case Rohatiner AZ et al. J Clin Oncol. 2007;25:2554-2559. Case Discussion Continued: • Our patient (now 64 years old) with follicular, grade 2 NHL with local disease status post R-CHOP × 6 cycles, relapsed 2.5 years later, and received bendamustine + rituximab; he had a partial response for 15 months and now presents with 2-cm diffuse LAN, asymptomatic • Platelet count is 90,000, and bone marrow biopsy shows 30% involvement of the intertrabecular space with FL cells; LDH is normal, as are blood chemistries • He is interested in nonchemotherapeutic and/or investigational approaches LAN = Lymphadenopathy ARS Question 3 Please record your answer on your evaluation form now. The preferred management for this patient is: A. Novel anti-CD20 antibody B. Lenalidomide C. Proteosome inhibitor D. PI3 kinase inhibitor E. Bruton’s tyrosine kinase (Btk) inhibitor F. RIT G. RICE or similar regimen, followed by ASCT ARS Question 3 The preferred management for this patient is: A. Novel anti-CD20 antibody B. Lenalidomide C. Proteosome inhibitor D. PI3 kinase inhibitor E. Bruton’s tyrosine kinase (Btk) inhibitor F. RIT G. RICE or similar regimen, followed by ASCT All the answers except RIT are reasonable options for the management of this patient. RIT is not a reasonable option for a Patient with a platelet count of 90,000 and 30% marrow involvement. Comparison of Novel Anti-CD20s Antibody Specificity Activity (vs Rituximab) Additional Features Type Isotype CDR CDC ADCC Apoptosis Ofatumumab I IgGI Human +++ = = Binds small extracellular part of CD20; completely human; slower off-rate PRO131921 I IgGI Humanized = ++ = Enhanced affinity for FcγRII Veltuzumab I IgGI Humanized =/+ = = Slower off-rate AME-133 I IgGI Humanized = + = Enhanced affinity for CD20 Tositumomab II IgG2a Murine - = ++ Bound to radio-isotopes GA-101 II IgGr Humanized - +++ +++ High affinity for FcγRII; strong induction of apoptosis Ig = immunoglobulin; CDC = complement-dependent cytotoxicity; ADCC = antibody-dependent cellular cytotoxicity. Adapted from Van Meerten T, Hagenbeek A. Neth J Med. 2009;67:251-259. Ofatumumab Ofatumumab binding site Rituximab binding site • Human CD20 monoclonal antibody that binds to membrane-proximal epitope encompassing both the small loop and large loop of CD201,2 • Approved in refractory CLL • Phase I/II study in relapsed or refractory FL3 – ORR: 42% – Median duration of response: 30 months – ORR in prior rituximab-treated patients: 64% 1. Teeling et al. J Immunol .2006;177:362. 2. Teeling JL et al. Blood. 2004;104:1793-1800. 3. Hagenbeek A et al. Blood. 2008;111(12):5486-5495. CLL = chronic lymphocytic leukemia. Ofatumumab in Relapsed/Refractory FL— Response Rates Number of patients (%) 100 80 ORR 60 13% 40 10% 11% 20 95% CI 0 Ofatumumab 500 mg (N=30) Ofatumumab 1000 mg (N=86) All patients (N=116) • 87% and 91% of patients in the 500-mg and 1000-mg groups, respectively, completed all 8 infusions of ofatumumab • No difference in primary endpoint (ORR) between the 500-mg and 1000-mg groups; thus, 2 groups were combined for secondary endpoint analyses Hagenbeek et al, ASH 2009. GA101: Mechanisms Of Action—Type I Versus Type II Antibodies Increased direct cell death Increased ADCC Unique type II epitope and elbow-hinge modification Via increased affinity to the “ADCC receptor” FcgRIIIA Effector cell B cell CD20 Lower CDC activity FcgRIIIa Due to recognition of type II epitope Complement Open-label, Phase II, Randomized Study of GA101 vs Rituximab Rituximab 375 mg/m2 IV qwk × 4 GA101 1000 mg IV qwk × 4 25 months after C4D1 MAINTENANCE Rituximab 375 mg/m2 IV q2mo × 12 End of maintenance visit - Relapsed CD20+ iNHL - Prior response ≥6 months to last rituximab regimen R A N D O M I Z E End of induction visit INDUCTION +28–42 days after C4D1 GA101 1000 mg IV q2mo × 12 PD discontinue treatment CT Sehn et al. ASH 2011. CT CT CT 1 3 6 CT 12 Time (mo) CT 18 CT 25 GA101 Versus Rituximab: Best Overall Response by in FL Patients Response, n (%) Rituximab (n = 75) GA101 (n = 74) 35 (46.7) 45 (60.8) CR/CRu 15 (20.0) 20 (27.0) PR 20 (26.7) 25 (33.8) ORR Difference in ORR, % [95% CI] P value (1-sided, χ2 test) Sehn et al. ASH 2011. 14.1 [–2.5; 30.8] .04 PFS Assessed by Investigators in FL Patients Survival probability Randomized treatment 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 Rituximab GA101 Hazard ratio [95% CI]: 1.1 [0.6; 1.8] 0 3 6 9 12 15 18 21 24 27 3 4 0 2 0 0 0 0 Study month Patients at risk, n Rituximab 75 GA101 74 Patients with an event, n (%) Median time to event, mo 95% CI for median time to event Sehn et al. ASH 2011. 63 61 56 55 48 45 20 26 15 19 Rituximab (n = 75) GA101 (n = 74) 26 (34.7) 29 (39.2) 17.4 17.3 [11.8; – ] [13.0; – ] IMIDs in Lymphoid Malignancies Thalidomide’s Various Effects in Myeloma • IMID effects – Inhibits TNF – Inhibits angiogenesis (bFGP, VEGF) – Stimulates T cells (CD8+) – Inhibits IL-12 – Induces apoptosis – Alters cytokines – Affects stromal cells – Inhibits prosurvival factors (Akt) Cool RM et al. Pharmacotherapy. 2002;22:1019; D’Amato RJ et al. Proc Natl Acad Sci U S A. 1994;91:4082; Meierhofer C et al. BioDrugs. 2001;15:681; Thalidomide’s various effects in myeloma [figure]. Available at: http://www.multiplemyeloma.org/treatments/3.04.asp; Weber D et al. J Clin Oncol. 2003;21:16; Bartlett JB et al. Nature Reviews/Cancer. 2004;4:314. Lenalidomide for Relapsed/Refractory Indolent Lymphoma Study No. of Pts Regimen Efficacy Witzig et al 43 Lenalidomide monotherapy ORR: 23%, CR: 7% PFS: 4.4 mo; DOR >16.5 mo Dutia et al 16 Lenalidomide + R (R2) ORR: 75%, CR: 31% PFS: 12 mo Ahmadi et al 15 Lenalidomide, dexamethasone, and R ORR: 53%, CR: 33% PFS: 86% at 10.9 mo PFS for Patients Receiving Lenalidomide + R 100 Survival (%) 80 60 40 20 0 0 5 10 15 Time (mo) 20 Witzig. J Clin Oncol. 2009;27:5404; Dutia. ASH. 2009 (abstr 1679); Ahmadi. ASH. 2009 (abstr 1700). Single-agent Bortezomib in Relapsed Indolent Lymphoma Study O’Connor, 2005 Goy, 2005 Strauss, 2006 Subtype Evaluable Patients (N) CR/CRu PR OR FL 9 1/1 5 7 (77%) MZL 2 0 2 2 (100%) SLL 3 0 0 0 FL 5 0/1 0 1 (20%) SLL 4 1/0 0 1 (25%) WM 2 0 1 1 (50%) FL 11 0 2 2 (18%) WM 5 0 2 2 (40%) WM = Waldenström’s macroglobulinemia. O’Connor OA et al. J Clin Oncol. 2005;23:676-684. Goy A. J Clin Oncol. 2005;23:667. Strauss. J Clin Oncol. 2006;24:2105. Promising New Agents in FL • Antibodies – New anti-CD20 – Anti-CD22, CD80, CD30, CD40, other targets • Immunostimulatory molecules • Other categories – Proteosome inhibitors – mTOR inhibitors – PI3K inhibitors (CAL-101) – IMIDs (lenalidomide) – Btk inhibitors (PCI-32765) mTOR = mammalian target of rapamycin; PI3 = phosphoinositide-3 kinase. B-cell Receptor Signalling and Inhibition in B-cell Malignancies Stevenson FK et al. Blood. 2011;118:4313-4320. CAL-101 Is an Orally Bioavailable Small Molecule That Inhibits PI3K Delta Potently and Selectively CAL-101 Alpha Beta Gamma Delta Cell-based Activity PDGF-induced pAKT LPA-induced pAKT fMLP-induced CD63+ FcR1-induced CD63+ EC50 (nM) >20,000 1900 3000 8 Class I PI3K Isoform • Selectivity relative to Class I PI3K isoforms involved in insulin signaling and other physiological functions • No off-target activity against Class II or III PI3K, mTOR, or DNA-PK • No off-target activity seen in screen of >350 protein kinases (Ambit KINOMEscan™) Lannutti. Blood. 2011. Single-agent CAL-101 Has Significant Antitumor Activity in Patients With Indolent NHL and CLL Best On-Treatment Change in Tumor Size (ITT Analysis) +100 % Change in Lymph Node Area +75 +50 +25 iNHLa CLLa (N=30) (N=54) 0 -25 -50b -75 -100 a Patients receiving single-agent CAL-101 at starting doses ranging from 50 to 350 mg/dose BID Inevaluable (patients without a follow-up tumor assessment) b Criterion for nodal response [Cheson 2007, Hallek 2008] Kahl, ASH 2010 (abstract 1777); Furman, ASH 2010 (abstract 55). Combination Therapy Had Effects on Lymphadenopathy in All Evaluable Patients With Indolent NHL Receiving CAL-101 + B or R Change in Lymph Node Area, % Best On-Treatment Change in Tumor Size +100 +75 +50 +25 CAL-101 + B (N=15) 0 CAL-101 + R (N=12) -25 -50a -75 -100 Inevaluable (patients without a follow-up tumor assessment) a Criterion for response [Cheson 2007] Leonard et al. ICML Lugano 2011. Phase I Study of the Btk Inhibitor, PCI-32765, in Relapsed/Refractory B-cell Malignancies • Btk is a downstream mediator of B-cell receptor signaling • Dosing: 1.25 mg/kg/d, with escalation to 2.5, 5.0, 8.3, 12.5, 17.5 mg/kg • 29 patients have been enrolled on cohorts 1-4 (12 FL, 7 CLL/SLL, 4 DLBCL, 4 MCL, 2 MZL) • One DLT (neutropenia); most AEs have been less than grade 2 Response Patients (n = 19) ORR 42% CR 1 (SLL) PR 7 (4 CLL/SLL, 2 MCL, 1 FL) DLBCL = diffuse large B-cell lymphoma; DLT = dose-limiting toxicity; AEs = adverse events. Advani et al. ASCO 2010 (abstract 8012). One Approach to Treat a Patient With Recurrent FL Post First-line Chemotherapy Needing Treatment • Late relapse (>2 years or so) – Many options; decision based on tolerability/efficacy balance • Early relapse (<2 years or so) – – – – Chemotherapy RIT SCT Novel/investigational agents Managing Patients with Mantle Cell Non-Hodgkin Lymphoma Case 2 • 56-year-old male who presents with diffuse lymphadenopathy and splenomegaly • Biopsy shows diffuse MCL • MIPI score: intermediate • He received R-hyperCVAD/MTX/cytarabine, followed by ASCT • Relapse in same areas 8 years later MIPI = Mantle Cell International Prognostic Index; R-hyperCVAD = rituximab–hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; MTX = methotrexate. CT Scan at Diagnosis Case Study: Pathology MCL showing germinal center overrun by broadened mantle zone in lymph node The MIPI • 4 factors independently associated with OS Age PS LDH WBC • Risk distribution – Low: 44% (score: <5.7) – Intermediate: 35% (score: 5.7-6.2) – High: 21% (score: >6.2) 1.0 0.8 Probability of OS – – – – Survival After Diagnosis by MIPI 0.6 0.4 Low, median not reached Int, median: 51 High, median: 29 0.2 0 0 WBC = white blood count; ECOG = Eastern Cooperative Oncology Group; ULN = upper limit of normal. Hoster E et al. Blood. 2008;111:558-565. 12 24 36 48 60 72 84 96 mo Formula for calculating MIPI: [0.03535 x age (years)] + 0.6978 (if ECOG > 1) + [1.367 × log10(LDH/ULN)] + [0.9393 × log10(WBC count)] Simplified MIPI • 0-3 points applied for each prognostic factor; sum reflects MIPI score - Low risk: 0-3 points - Intermediate risk: 4-5 points - High risk: 6-11 points Points Age (y) ECOG PS LDH/ULN WBC (cells/mm3) 0 <50 0-1 <0.67 <6700 1 50-59 -- 0.67-0.99 6700-9999 2 60-69 2-4 1.00-1.49 10,000-14,999 3 ≥70 -- ≥1.50 ≥15,000 Hoster E et al. Blood. 2008;111:558-565. MCL • 5%-10% of B-cell NHLs, with moderately aggressive course • 74% male; median age, 63 years • >80% stage III/IV, including marrow involvement • Extranodal sites common: lymphomatous polyposis, gastrointestinal, soft tissue, or leukemic phase • Prognosis poor: chemoresponsive, but median survival 30 months with CHOP-type chemotherapy Fisher RI et al. Hematology Am Soc Hematol Educ Program. 2004;221-236. Front-line Chemotherapy Options for Patients With MCL R-CHOP Modified HyperCVAD R-CHOP/RIT R-Bendamustine Less intensive vs R-CHOP/ASCT R-HyperCVAD/MTX/Ara-C R-HyperCVAD/MTX/Ara-C/ASCT Nordic More intensive ARS Question 4 Please record your answer on your evaluation form now. What second-line therapy would you choose for this patient (now age 64 years)? A. Purine analogue–based regimen B. Bortezomib ± rituximab C. Lenalidomide D. Bendamustine-based regimen (BR, BVR) E. Experimental therapy BR= Bendamustine + rituximab; BVR= Bendamustine + bortezomib + rituximab ARS Question 4 What second-line therapy would you choose for this patient (now age 64 years)? A. Purine analogue–based regimen B. Bortezomib ± rituximab C. Lenalidomide D. Bendamustine-based regimen (BR, BVR) E. Experimental therapy All the answers are reasonable choices for this patient. The patient was put on a bendamustine-based regimen. BR= Bendamustine + rituximab; BVR= Bendamustine + bortezomib + rituximab Recommendations for Second-line Therapy • Suggested regimens or radiotherapy or clinical trial Suggested Regimens Bendamustine ± R Bortezomib ± R Cladribine + R Fludarabine-based regimens: FC ± R, FCMR, FMR Lenalidomide ± R PCR PEPC ± R • Second-line consideration of allogeneic transplantation F = fludarabine; C = cyclophosphamide’ M = mitoxantrone; R = rituximab; P = pentostatin; PEPC = prednisone, etoposide, procarbazine, cyclophosphamide. NCCN clinical practice guidelines: non-Hodgkin lymphomas. 2011. Second-line Therapy for MCL • Purine analogue–based regimen – ORR: 50% to 70% • Bortezomib ± rituximab – ORR: 30% to 50% • Lenalidomide – ORR: 30% to 50% • Bendamustine-based regimen (BR, BVR) – ORR: 50% to 70% • Experimental therapy Bendamustine, Bortezomib, and Rituximab • Phase II, multicenter study • Patient population: relapsed, indolent B-cell, or mantle cell NHL • Exclusion: prior ASCT or RIT within 4 months; prior allogeneic SCT at any time • Schema: 28-day cycle Day 1 Day 4 Bendamustine 90 mg/m2 X X Rituximab 375 mg/m2 X Bortezomib 1.3 mg/m2 X Friedberg et al. Blood. 117 (10): 2801-12. X Day 8 Day 11 X X Response by Histology Category Response Rate % (95% CI) Follicular NHL 93% (69-99%) Mantle cell 71% (36-92%) Friedberg et al. Blood. 117 (10): 2801-12. ARS Question 5 Please record your answer on your evaluation form now. What are the major acute toxicities associated with the BVR regimen? A. Anemia, thrombocytopenia, neutropenia B. Peripheral neuropathy C. Secondary malignancies D. A and B E. A, B, and C ARS Question 5 What are the major acute toxicities associated with the BVR regimen? A. Anemia, thrombocytopenia, neutropenia B. Peripheral neuropathy C. Secondary malignancies D. A and B E. A, B, and C Monitoring After BVR • Major acute toxicities – Anemia, thrombocytopenia, neutropenia – Peripheral neuropathy – Nausea/vomiting, diarrhea • Long-term toxicities (>1 month off chemotherapy) – Prolonged thrombocytopenia – Peripheral neuropathy Case Discussion Continued: Relapse #2 • The patient stays in partial remission for 9 months and then has a progression in multiple lymph node sites • He is considering several clinical trials – Lenalidamide ± rituximab – Cal-101 – PCI-32765 Rituximab in Combination With Lenalidomide in MCL* • Phase I/II study in relapsed/refractory MCL • MTD: lenalidomide 20 mg plus rituximab 375 mg/m2 Objective Response % (n/N) Overall response 70% (7/10) CR PR SD 30% (3/10) 40% (4/10) 10% (1/10) Response duration 8 months *Wang M et al. ASH 2009: Abstract 2719. CAL-101 Is an Orally Bioavailable Small Molecule That Inhibits PI3K Delta Potently and Selectively CAL-101 Alpha Beta Gamma Delta Cell-based Activity PDGF-induced pAKT LPA-induced pAKT fMLP-induced CD63+ FcR1-induced CD63+ EC50 (nM) >20,000 1900 3000 8 Class I PI3K Isoform • Selectivity relative to Class I PI3K isoforms involved in insulin signaling and other physiological functions • No off-target activity against Class II or III PI3K, mTOR, or DNA-PK • No off-target activity seen in screen of >350 protein kinases (Ambit KINOMEscan™) Lannutti. Blood. 2011. Cal-101 in B-cell Lymphoma Best Response Best on-treatment change in tumor size (ITT analysis) % Change in Lymph Node Area +100 +75 +50 +25 MCL (n = 21) iNHL (n = 30) 0 -25 -50* -75 -100 Inevaluable (patients without a follow-up tumour assessment; includes two patients with LPL with no adenopathy) * Criterion for response [Cheson 2007, Hallek 2008] Kahl B et al. Blood (ASH Annual Meeting Abstracts). 2010;116:1777. PCI-32765 Novel Small-molecule Btk Inhibitor • Forms a specific and irreversible bond with cysteine-481 in Btk • Potent Btk inhibition O NH 2 – N N N IC50 = 0.5 nM • Orally available N • N O Once-daily dosing results in 24hour sustained target inhibition Best Response 71% 69% 65% CR 16% 16% 15% PR SD PD 16% 20% 13% 55% BTZ-naïve (n = 31) BTZ= Bortezomib *Wang et al. ASH 2011; Abstract 442 18% 15% 50% BTZ-exposed (n = 20) 53% Total (n = 51) Second-line Therapy Options for MCL • Evaluate age and overall condition of the patient— transplantation-eligible? • Standard options – Purine analogue – Bendamustine – Bortezomib – Autologous or allogeneic SCT in CR2 • Investigational options – Lenalidamide – BCR pathway agents Thank you for joining us today! Please remember to turn in your evaluation form. Your participation will help shape future CME activities.