Eosinophilic GI Disorders
advertisement
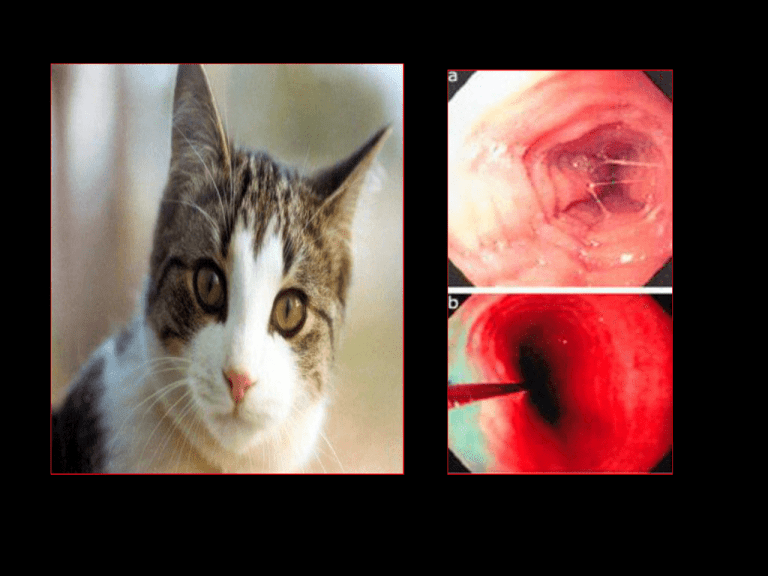
Esophageal Eosinophilia Is it due to...? • severe GERD – Intraepithelial eos correlate w/reflux esophagitis – but.. • Normal esophageal pH studies • Poor response to acid blockade • subset of eosinophilic gastroenteritis • manifestation of IBD or other autoimmune disorder • a new separate disease Eosinophilic Esophagitis • Isolated eosinophilic infiltration of esophageal mucosa • Digestive symptoms – Dyspepsia, dysphagia – Can mimic GE reflux – Unresponsive to acid suppression therapy • Emerging disease – Increasing incidence Chronology • 1953: Schatzki’s ring reported – Possible association with EE • 1970s: “Felinized” esophagus reported – Multiple concentric rings attributed to GERD or congenital anomaly • 1978: First report of “eosinophilic esophagitis” as separate entity – Landres et al, Gastroenterology 74:1298. • 1982: Correlation established between eos and reflux esophagitis • 1995: Kelly and Sampson demonstrate link between persistent EE and dietary antigens – Improvement with an elemental formula; Gastroenterology 109:1503-12. • 1998: First reports of successful treatment of EE with oral or topical corticosteroids • 2003: First study of natural history of EE in adults published • 2006: First randomized controlled trial of pharmacologic therapy in EE (swallowed fluticasone) published • 2006: First Int’l. Gastrointestinal Eosinophil Research Symposium (FIGERS) begins developing guidelines for diagnosis and management of EE in children Epidemiology • PREVALENCE – 1.5-3/10000 adults – 4.3/10000 children • Incidence rising – Approaching that of IBD • Family history – 6.8% of patients • Male predilection Noel R et al. N Engl J Med. 2004: 351:940-41. Clinical Features of EE CHILD Vomiting Heartburn ADOLESCENT Heartburn Dysphagia ADULT Dysphagia Stricture Yan B, Shaffer EA. World J Gastroenterol. 2006 Pediatric Series • N = 381 – Age 9 +/- 3 y • 2 cases in 1994, vs. • 72 cases reported in 2003 • 66% male • 85% GER sx – 18% dysphagia • Endoscopy – Normal in 32% • Despite clearly abnormal histology Liacouras CA et al. Clin Gastroenterol Hepatol. 2005; 3:1198. • N = 103 – Only 3% identified prior to 2000 • Atopic history – – – – Rhinoconj. 57% Food allergy sx 46% Wheezing 37% Fam hx atopy 74% • Age-dependent clinical features Noel R et al. N Engl J Med. 2004; 351:940-41. Clinical Features of Pediatric EE Noel R et al. N Engl J Med. 2004: 351:940-41. Represents median age of presentation EE vs. GER Diagnosis of EE • Requires endoscopy and histology – Strictly defined as dense eosinophilia confined to the esophagus – Eosinophilia also in stomach, small bowel, colon may be due to another EGID, IBD, parasitic or fungal infection, connective tissue disease, neoplasm • Adjunctive modalities – – – – – Contrast UGI series: eval. dysphagia Esophageal pH-metry: exclude acid GER CBC/d: periph. eosinophil count Serum IgE level Future: biomarkers Endoscopy Normal esophagus EE Linear furrowing Eosinophilic microabscesses Furuta GT, Straumann A. Aliment Pharmacol Ther. 2006;24:173-82. Normal “Crepe paper” esophagus EE Trachealization Contrast UGI series Fox VL et al, Gastrointestinal Endosc. 2002 EGD: Mucosal pallor UGI: Stricture EUS: Submucosal thickening Fox VL et al, Gastrointestinal Endosc. 2002 Histology • Eosinophils in the esophagus are never normal • Eosinophil count (eos/hpf) alone is not enough to establish dx of EE • >15 eos/hpf suggests EE in the proper clinical context • Pathology report should quantify eos in the most dense field • Multiple biopsies should be taken from distal and proximal sites in esophagus • GERD esophagitis is typically localized to distal esophagus • Inflammation may have “patchy” distribution Ruchelli E, Antonioli D, FIGERS/NASPGHAN Annual Meeting 2006 . Proposed Biomarkers • Non-invasive • Reproducible and predictive • Based on pathophysiology – Sputum eosinophils – Serum CD23 levels – Plasma eotaxin-3 and eosinophil-derived neurotoxin (EDN) levels • Correlated strongly, along with peripheral eosinophil count, with mean esophageal eosinophil density* – mRNA or gene microarray for eotaxins and cytokines (IL-5, IL-13, RANTES) Gupta SK. FIGERS 2006. *Konikoff MR et al. Clin Gastroenterol Hepatol. 2006; 4:1328-36. Pathogenesis Present understanding: Chronic allergy “An immune disorder that results from a mixed allergic response” to dietary and possibly other environmental antigens* Kay AB. New Engl J Med. 2001 Th-2 response IL-5 key for eosinophil differentiation, activation Eotaxins, IL-4, IL-13 recruit eos to GI tract *Markowitz JE, Liacouras CA. Dig Liver Dis. 2006; 38:251-53. Pathogenesis Rothenberg ME. New Engl J Med. 1998 • Once in GI tract, eos release eotaxins, IL-5, GM-CSF, PAF and attract more eos • Eos cause local inflammation by releasing MBP, cytotoxic granule contents, more cytokines • Ongoing inflammation can lead to fibrosis, stenosis, morphologic alteration Role of Environmental Allergens • • • • Seasonal exacerbation in some cases Association with pollen allergy Atopic background of many EE patients Experimental EE (mouse models) – Respiratory allergens induced EE while oral or intragastric ones did not – Intratracheal IL-13 induced EE – Deficiency in IL-5, eotaxin-3 and its receptor, and STAT6 protected mice vs. EE Mishra A et al. J Clin Invest. 2001; 107:83-90. Rothenberg ME. FIGERS and NASPGHAN Annual Meeting 2006. Blanchard C et al. J Allergy Clin Immunol. 2006; 118: 1054-9. Rothenberg ME. J Allergy Clin Immunol. 2004; 113:11-28. Natural History • In general, the disease “stays around” – No mortality but persistent morbidity – No evidence of dysplasia or malignant transformation • Complications of untreated EE – – – – – – – Esophageal stricture Food impaction Sliding hiatal hernia (esoph. shortening) Tracheal edema Subglottic stenosis Superinfection with Candida or CMV Risk of emesis-induced or endoscopic perforation Natural History • Based on limited data – 30 adult patients followed for up to 11 years* • No adverse impact on nutritional status • No worsening of sx, but no histologic improvement – 24 pediatric patients who refused tx or were lost to follow-up** • Mean follow-up 6 yrs later • All had persistent eosinophilia • 20 who had presented with GER sx came back with dysphagia – N=89 (CCH 8 yr retrospective): of 66% of the patients who had initial resolution, 79% later relapsed*** • Chronic disease at best, progressive at worst – Progression: esophagitisringssmall caliberpermanent fibrosis and stricture • • • Histologic relapse off therapy is common Absence of sx does not predict absence of inflammation Effectively treated patients have not been observed to develop dysphagia or fibrosis * Straumann A et al. Gastroenterology 2003. ** Liacouras CA, Putnam P. FIGERS 2006. ***Assa’d A et al. J Allergy Clin Immunol. 2007. Treatment Options Intervention Advantages Drawbacks Cromolyn Safe Limited efficacy Montelukast Convenient Clinical response without histologic improvement Topical corticosteroid Efficacious, easy Relapse off therapy Potential adverse effects Systemic corticosteroid Efficacious, easy Relapse off therapy Established adverse effects Elimination diet Non-pharmacologic alternative in select pts Difficult, may induce food aversions Efficacy variable Elemental diet Efficacious May prevent relapse Very difficult Usually requires feeding tube • 31 children with EE • Fluticasone 880 mcg/d PO X 3 months – Induced remission (lowered peak eos ct to <1) – Improved endoscopic and histologic features – Reduced vomiting – Reduced esoph. CD8+ T cells and mast cells – Was safe • Responders – Non-allergic (neg. SPT) – Younger, shorter, lighter Dietary Therapy – Kelly & Sampson, 1995 • N = 10 children • Strict AA formula X 6 w – 100% clin/histol response – 80% remission – Markowitz et al., 2003 • N = 51 children, 48/51 responded to AA-based formula (Neocate 1+) – NG tube in all but 3 – Liacouras et al., 2006 • N = 381 over 10 years • 172 tx’d with AA-based formula – 128 required NGT – Eos/hpf » Pre-diet: 38.7 » Post: 1.1 • 75 tx’d with elimination diet based on SPT/APT – Spergel et al., 2005 • Elim. Diet based on SPT/APT – Kagalwalla et al., 2006 • Six food elimination diet – CMP, soy, egg, wheat, peanut, seafood Dietary Therapy 100 90 80 70 60 50 40 30 20 10 0 95 % responders 77 74 Post-diet eos/hpf 13.8 12.8 1.1 Six Food Elim. SPT/APTDirected Kagalwalla N = 35 Spergel N = 146 Elemental Liacouras N = 160 Kagalwalla A et al. Clin Gastroenterol Hepatol. 2006; 4:1077-1102. Spergel JM et al. Ann Allergy Asthma Immunol. 2005; 95:336-43. Liacouras CA et al. Clin. Gastroenterol. Hepatol. 2006; 3:1198. Role of SPT/APT Spergel JM et al., 2007 • Combination testing – Can identify correct elimination diet in 70% • Resolution of sx/bx • Specific foods were definitely identified as the cause of EE in 39/146 – On elimination bx normalized – Reintroduction of the causative foods » relapse of symptoms » Bx: return of eosinophilic inflammation • The combination of the 2 testing methods had an excellent NPV (88% to 100%) – for all foods except milk, which was very low at 41% Khan S, Henderson N (2002) Current Treatment Options in Gastroenterologyg • Do children with EE grow up to be adults with EE? • What is the role in EE pathogenesis of... – food and aeroallergen cross-reactivity? – IgE? • Is there a genetic or phenotypic difference between atopic and non-atopic EE? • What is the prevalence of EE among highly atopic pts? • What is the value of RAST, skin prick testing, and atopic patch testing in dx of EE? • Therapeutic prospective comparisons: – Elemental diet vs. elimination diet vs. corticosteroids • Should endpoint of treatment be... – clinical remission, OR... – normalization of histology? Paul Ehrlich 1854-1915 Eos GREEK GODDESS OF DAWN