CME_121914_Watson_HOs - St. Charles Health System
advertisement

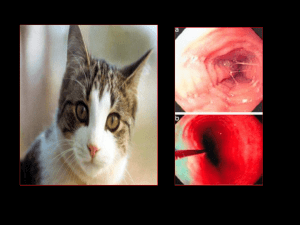
Eosinophilic Esophagitis No Relevant Disclosures Rabindra R Watson, MD Assistant Clinical Professor of Medicine Director, Career Development in Advanced Endoscopy Division of Digestive Diseases David Geffen School of Medicine Objectives • Discuss the rising prevalence of eosinophilic esophagitis (EoE) • Discuss the differentiation between EoE and gastroesophageal reflux disease • Discuss best treatment approaches for EoE Definition A primary clinicopathologic disorder of the esophagus, characterized by: Esophageal/Upper GI tract symptoms >15 intraepithelial eosinophils/HPF on esophageal biopsy Absence of GERD by either normal pH study or lack of response to high dose PPI therapy* Furuta GT, et al, Gastro 2007 Definition • "a chronic, immune/antigen-mediated, esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation" Names/Acronynms for EE Liacouras, et al, J Allergy Clin Immunol 2 Eosinophilic Esophagitis (EE or EoE) Primary Eosinophilic Esophagitis (PEE) Allergic Eosinophilic Esophagitis (AEE) Idiopathic Eosinophilic Esophagitis (IEE) Corrugated Esophagitis Allergic Esophagitis History of EE First described by Landres in 1978 Virtually no studies until the 1990’s Marked increase in publications over last 5 years Publications now number more than that for eosinophilic gastroenteritis Initially described in children, but now increasingly being recognized in adults Most studies single center with small numbers of patients Dellon et al, AJG, 2007, pp. 2300‐2313 Epidemiology EE>EG In Publication Numbers • Diagnosis (?incidence) rising • Straumann and Simon - 13.5 x increase in Switzerland over 16 years • Studies done in demographically stable area with consistent recording -> suggesting real increase not enhanced disease awareness • Regional variations • Urban > Rural • Regional • Incidence ~ 4 : 10,000 Spergel, et al, JPGN 2011;52: 300–30 Furuta et al, APT, 2006, pp. 173-182. History of the Eosinophil G ER D Number of Publications on EE But: Prevalence of GERD: 10-20% Spergel, et al, JPGN 2011;52: 300–306 EOS = Greek for “goddess of the dawn” 1879 - eosinophil named by Paul Ehrlich because cytoplasmic granules stained red with eosin (like the morning glow) Pathophysiology Pathophysiology – Interleukins Allergen-induced Often related to food ? aeroallergen(s) in minority of people Allergen (IgE) and non-allergic (non-IgE mediated mechanisms Why Esophagus? Allergen exposure Eosinophilic inflammation Exposure to Antigens Æ Th2 response Æ ÏIl-4, Il-5, Il-13 Influences Eosinophil activation in bone marrow Release of Eotaxin-1 from esophageal epithelial cells Dysphagia/foo d impaction Scarring (fibrosis) Potent promoter of eosinophil migration Gene expression Ï53x in children with EoE Blanchard C, et al. J Clin Invest 2006 Pathophysiology – Eosinophils Presentation Extracellular deposition of major basic protein Eosinophilic granules are toxic to intestinal epithelium Esophageal lymphocytes, mast cells, fibroblasts are increased Æ remodeling • Male predominance (65-71%) • Age 20-40’s (mean 33.5) • 52.8% with diagnosis of additional allergic condition • Dysphagia most common • 15% with EoE • History of food impaction in 35-54% Dellon ES, et al. Clin Gastro Hep; 2014 Prasad GA et al. Am J Gastro 2007 Mackenzie SH et al. Alim Pharm Therap 2008 Straumann A, et al. Clin Gastro Hep 2008 Symptoms Dysphagia to solid foods Food impaction Meat Heartburn (Refractory GERD) Bread Chest pain Abdominal pain Vomiting* Poor feeding* *seen in younger children only Differential Diagnosis Rice Rough-texture foods Liacouras, et al, J Allergy Clin Immunol 2011 Laboratory Studies Suggestive of EE Diagnostic Guidelines Clinical symptoms of esophageal dysfunction >= 15 Eosinophils in 1 high‐power field Lack of responsiveness to high‐dose proton pump inhibition (up to 2 mg/kg/day) or Normal pH monitoring of the distal esophagus Making the diagnosis Requires an upper endoscopy (EGD) and biopsies ≥2-3 biopsies each from upper and lower esophagus ≥15 eosinophils per section under microscope at high-level magnification Eosinophilic inflammation absent from stomach and small intestine Negative pH study – no acid reflux Eosinophilia 60% of children 5‐50% of of adults Elevated serum IgE levels Positive skin prick test Radioallergosorbent tests (+ in 40‐73%) Endoscopic findings • 80% • Rings • Linear furrows • Felinization • Stricture • White exudates Note: None of these are pathognomonic for EE Histology Variability in Histologic Criteria Review of literature through 2006 on EE 116 original articles, 39 abstracts, 69 reviews Results showed 10 different histologic definitions for EE (5-30 eosinophils/HPF) 35% of articles did not state diagnostic criteria 11% of original articles reported a high powered field area Eosinophil density per mm2 varied by factor of 23 Specific biopsy protocols reported in only 39% of articles Dellon et al, AJG, 2007, pp. 2300-2313. Diagnostic challenges: PPI-REE PPI-responsive esophageal eosinophilia (PPI-REE) Initially thought that EoE and acid reflux (GERD) were distinct entities ??? Exclude GERD by trial of PPIs or reflux testing EoE and GERD can co-exist Symptoms and EGD-findings suggestive of EoE Microscopic requirements for EoE Symptom and microscopic response to PPI +/- abnormal reflux testing Diagnostic challenges GERD predisposes to antigen exposure Decreased tight junction integrity, ?increase antigen exposure EoE may reduce LES pressure, promote lamina propria fibrosis Role of allergy evaluation Recommended to evaluate for concurrent atopic disorders Testing for immediate-type food, environmental allergies 40% of EoE pts have motility abnormalities PPIs are anti-inflammatory Antioxidants Ðcytokine production Ðadhesion molecule producition ÐIl-13, Il-4 stimulated eotaxin production Serum (IgE) Skin prick and atopy patch testing Usually not helpful for identifying EoErelated food allergies Sgouros SN, et al. Eur J Gastro Hep 2006 Therapeutic Goals Therapy PPI PPI Treatment Impact PPI PPI Therapeutic Response PPI PPI Treatment Options – PPIs Proton Pump Inhibitors Topical Steroids Omeprazole (Prilosec) Lansoprazole (Prevacid) Pantoprazole (Protonix) Rabeprazole (AcipHex) Esomeprazole (Nexium) Dexlansoprazole (Dexilant) Mainstay of treatment Inhaler (fluticasone) – swallowed instead of inhaled Viscous (budesonide) – mix with sugar substitute May have anti-inflammatory properties 33-50% of patients with EoE features respond Twice a day dosing x8 weeks to see if PPI-REE Topical Steroid Therapy Topical Steroids Twice a day for 8-12 weeks Nothing to eat/drink for 30 minutes after swallowing medication Rinse, gargle and spit to clear residual medicine from mouth Liacouras et al 1% oral yeast infection (thrush) 5% esophageal yeast (usually asymptomatic and probably not significant) Faubion et al Arora et al Topical Steroid Therapy 26 patients; 19 received fluticasone propionate 250 ug/puff; 2 puffs BID x 4 weeks 4 week trial of topical steroids 21/21 children with near total symptom relief in 1 week Side effects uncommon Complete symptoms relief in 1 week. 21 patients treated for 6 weeks Complete symptom relief for 4 months 3 patients with relapse afer 4 months About 1/2 of patients relapse within 12‐18 mos Topical Therapy Improves Symptoms and Histology Symptoms = Dysphagia, CP, Heartburn, Regurgiation, Vomiting, Abdominal Pain All treated patients with symptom improvement and histologic improvement 11 became asymptomatic Mean symptom score went from 5.4 to 0.7 14/19 with recurrent symptoms at 3 months 3 patients developed candidiasis Remedios et al, GIE, 2006, pp. 3-1 Remedios, GIE, 2006, pp. 3-1 Budesonide for EoE Budesonide for EoE RCT with placebo control of 1mg BID x 15d (N=36 total) Primary outcome = # of mucosal eosinophils Significant decrease in eosinophils (68.5 -> 5.5 hpf in tx group); dysphagia improved as well (5.61 to 2.22); p<0.0001 for both comparisons; no change in placebo group Also endoscopic improvement seen and no adverse RCT with placebo control of 1 or 2 (< or > 5 ft) mg QD viscous budesonide x 3 mos (N=15 budesonide; N=9 placebo) Primary outcome = # of mucosal eosinophils Significant decrease in eosinophils (66.7 -> 4.8 hpf in tx group); eosinophilia decreased throughout esophagus Also endoscopic, symptom, and histologic improvement Oral Steroids Prednisone May help if poor response to topical steroids or in patients who need rapid improvement in symptoms Not commonly used Numerous side effects from long-term use Dietary Elimination Elemental (amino acid based) diet Most effective (>95% of patients respond) Elemental formula is costly Usually administered by feeding tube (not palatable) Elimination based on allergy testing Low predictive value for identifying EoEassociated allergen(s) Markowitz, AJG, 2003 Elemental Diet Therapy in Adults 18 adults Eosinophil count decreased from 54 to 10/hpf 72% with near complete response (<10 eos/hpf) 38% failed to adhere to diet No symptom improvement Eosinophil count increased back to previous in 3-7 days Dietary Elimination Six-food elimination Wheat, dairy (including milk proteins), soy, nuts, egg, seafood 64-94% resolution (depending on parameter assessed) Acceptable first-line therapy for EoE > Petersen et al, Am Jl Gastro, 2013, pp 1-8 > = > Markowitz, AJG, 2003 Issues with SFED Six-food elimination diet (SFED) Avoid foods listed in the SFED x6 weeks Assess if endpoints met Symptom resolution Improvement of endoscopic appearance Appropriate reduction in esophageal eosinophils If endpoints met, reintroduce food groups one at a time every 2 weeks to identify trigger(s) Wheat (60%) Dairy (50%) Soy, nuts (10% each) Eggs (5%) > > Some patients’ symptoms may not occur so frequently = > Up to ~35% of patients may not respond to SFED Another food? (rice, corn, legumes) Can be time consuming and still at “square one” Can be difficult to eliminate all 6 food groups Four-food elimination maybe as effective (nuts and seafood OK) Wh t b t j t li i ti h t d d i (t i Leukotriene D4 Receptor Inhibitors Six food elimination diet (SFED) Longer duration on SFED before recognizing symptom response Longer duration for each food reintroduction before determining whether each food is a trigger 50 adults 6 weeks Clinicopathological remission with SFED Eosinophilia returned when diet liberalized Cromolyn - mast cell inhibitor Montelukast Single study of 14 pts showed no benefit Has been used to treat asthma Needs to be given daily rather than in a pulse manner Attwood et al 8 patients treated with 10 mg/day Adjusted dose up to 100 mg/day 7/8 with symptom resolution, although not histologic resolution Adverse events: nausea and myalgia 75% recurred within 3 weeks of stopping drug Gonsalves et al, Gastroenterology 2012 Biological- Reslizumab-(anti-IL-5 antibody) Interleukin‐5 Inhibitors Mepolizumab - IL-5 monoclonal antibody Reported for adult EoE (Garrett, J. All Clin Imm, 2004) 4 patients with hypereosinophilic syndromes 1 patient with EE Unresponsive to diet, topical/oral steroids Given study drug 3 x at 10 mg/kg every 4 wks Symptomatic, endoscopic, and histologic improvement seen. 226 children (mean age-12 +/1 4) 3 doses and placebo 12 weeks Histological response with treatment Treatment and placebo symptom response Spergel JM et al, J Allerg Clin Immunol 2012 Endoscopic Dilation Breaks up scar tissue Useful for treating very tight strictures Feasible in absence of prior medical therapy Effective for quick relief of dysphagia Does not treat the underlying inflammation Risks Indications for Dilation Chest pain (75%) – usually resolves within 2-3 days Clinically significant bleeding (only 1 case report in literature) Perforation (0 3% in academic medical centers) Known EE with dysphagia that fails to respond to medical therapy Severely symptomatic strictures Defer dilation at initial endoscopy if EE is suspected About 80% will have immediate response to dilation Symptoms tend to recur, but relief usually lasts at least 3-8 months Endoscopic Dilation Dilation is Safe – But Painful 207 dilations Mean recurrence in dysphagia at 23 mos in dilation only group and 20 mos in dilation and med tx (p=NS) No perforations; 74% with chest pain Symptom relief despite no change in eosinophil counts Maintenance Stopping therapy will lead to relapse in >90% of patients by 9 months Prognosis Maintenance therapies Indicated particularly for patients with highgrade strictures and rapid relapse Topical steroids used at 25-50% of the initial dose Long-term elimination of dietary trigger(s) On-demand esophageal dilation Little data to date on this important issue Long term prognosis of pediatric EE unknown ? If similar to food allergy/atopic dermatitis which abate with age Strautman et al, Gastroenterology, 2003 Followed 30 adults with EE Mean f/u of 7.2 years Dysphagia persisted in 97% Strictures in 13/30 No progression of eosinophilic infiltration No malignancies/hypereosinophilic syndromes Only 3% with a major negative impact on activity Conclusions EoE is a chronic immune disorder of the leading to esophageal dysfunction Pathophysiology is unclear, ?asthma of esophagus Rising incidence in parallel with allergic disorders EoE and GERD can coexist: PPI trial Therapy is multifaceted: steroids, elimination, endoscopic dilation Insufficient data to determine long term outcomes Questions? "I wanted you to meet our endoscopy specialist before we miniaturized him." rwatson@mednet.ucla.edu