PowerPoint slides
advertisement
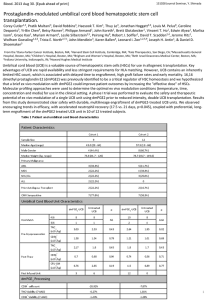
Circulating Progenitor Cells and Cardiovascular Disease Arshed A. Quyyumi, MD Professor of Medicine Emory Clinical Cardiovascular Research Institute (ECCRI) Emory University School of Medicine Atlanta, GA Grant support: National Institutes of Health, American Heart Association, Woodruff Fund, Emory Heart and Vascular Center, Sanofi Aventis, Novartis, Lilly, Pfizer, Forest, Amgen, Genzyme Advisory Boards: Amorcyte/Neostem, Genway/Firstmark Soteria Stemedica Circulating progenitor cells CD34+ cell populations Circulating progenitor cells and gender Circulating progenitor cells and ischemic syndromes Circulating progenitor cells and CVD outcomes CD34+ cells as therapy for vascular diseases Adult Bone Marrow Stem Cell Plasticity Neural cells Epidermal cells Ectodermal Progenitor Cells Endothelial Progenitor Cells Blood cells Mesodermal Progenitor Cells Hematopoeitic cells Bone Marrow Stem Cells Endodermal Progenitor Cells Hepatocytes Resident stem cells: Heart, skeletal muscle, Adipose tissue, brain, Lung etc. Stromal or Mesenchymal MAPC Osteocytes, Chondrocytes Myocytes (Skeletal) (Cardiac) 4 Circulating progenitor cells CD34+ cell populations Circulating progenitor cells and gender Circulating progenitor cells and ischemic syndromes Circulating progenitor cells and CVD outcomes CD34+ cells as therapy for vascular diseases Flow Cytometry 250K 105 200K 200K 104 <APC-A>: CD133 250K SSC-A SSC-A 31.8 150K 150K 100K 100K 0.113 10 3 10 2 35.2 0.585 62.4 1.85 0 50K 50K 0 0 0 50K 100K 150K FSC-A 200K Gated on MNCs CD45med 250K 2 3 0 10 10 10 <FITC-A>: CD34 4 Gated on CD34+ within MNCs 10 5 2 3 0 10 10 10 <PE-A>: VEGF-R2 4 10 Within CD34+ gate, 4 quadrants for marker CD133 and VEGF-2R 11 5 Fluorescent activated cell sorting (FACS) analysis for bone marrow derived progenitor cell populations CD34: Hematopoietic stem cell CD133: Immature progenitors CD133+/VEGF2R+: Differentiates between immature and mature endothelial PCs CD34+/CD133+/VEGF2R+: Presumed ‘EPC’ enriched CXCR4: Epitope that is associated with homing to areas of ischemia that express SDF-1 Peichev M et al Blood 2000; 95:952 Hirschi KK. et al ATVB 2008;28:1584 12 Cell Type: Isolated CD34⁺Cells Most Able to Improve Perfusion, Prevent Apoptosis and Rescue Hibernating Cardiomyocytes CD34⁺ Cells Exhibit Increased Potency and Safety for Therapeutic Neovascularization after AMI Compared with Total Mononuclear Cells in Nude Rats: PBS = Phosphate-buffered saline loMNCs = 5x10^5 MNC hiMNCs = contains 5x10^5 CD34+ cells within MNCs CD34+ = 5x10^5 CD34+ cells Capillary Density (perfusion) is greatest in CD34+ cell cohort, and this correlates with decreased incidence of fibrosis. Effect increases with dose. Kawamoto et al., Circulation 2006;114;2163-2169 13 Endothelial progenitor cell therapy for acute myocardial infarction Cell types: bone marrow mononuclear cells, CD34+, etc. CD34+ cells constitute 0.1 to 0.2% of bone marrow mononuclear cells • FDG labeled bone marrow mononuclear cells and injected intracoronary in post MI patients BM mononuclear cells • Uptake of BM mononuclear cells: 1.3 to 2.6% in MI region CD34+ cells • Uptake of CD34+ cells: 14-39% in MI region Hofmann et al Circ 2005;111:2198-2202 Circulating progenitor cells CD34+ cell populations Circulating progenitor cells and gender Circulating progenitor cells and ischemic syndromes Circulating progenitor cells and CVD outcomes CD34+ cells as therapy for vascular diseases Figure3: Age-related changes in circulating PC populations in men (green) and women (blue) Hematopoietic (top) and endothelial-enriched PCs (bottom) Men: r=-0.16, p=0.011 Women: r=-0.13, p=0.003 Men: r=-0.1, p=0.05 Women: r=-0.04, p=0.4 Men: r=-0.19, p=0.003 Women: r=-0.14, p=0.001 Men: r=-0.1, p=0.09 Women: r=-0.05, p=0.2 18 Circulating progenitor cells CD34+ cell populations Circulating progenitor cells and ischemic syndromes Circulating progenitor cells and CVD outcomes CD34+ cells as therapy for vascular diseases Circulating progenitor cells in acute coronary syndromes: comparison with stable CAD 90 ACS patients (mean age 65±15 yrs, 73% male, 10% STEMI, 76% NSTEMI, and 13% unstable angina) Blood samples were obtained at the time of cardiac catheterization for enumeration of CPCs as CD45dim cells using flow cytometry. An age- and gender-matched (1:2) cohort of stable CAD patients were randomly selected as a control group Comparison of VEGF2R-expressing CPCs between stable CAD and ACS categories * * * * Mental Stress Standardized public speaking task Role playing a difficult Mental Stress interpersonal situation where a close relative in a nursing home is being mistreated. Physical Stress Rest Rest Hemodynamic and electrocardiographic monitoring Mental stress Physical stress Results Effect of mental stress on acute mobilization of progenitor cells • MS challenge provoked an average 21% increase in the number of circulating CD34+/VEGF2R+/CXCR4+ cells Relationship between progenitor cells and ischemia during mental stress Patients with mental stress ischemia had higher circulating number of CXCR4-expressing cells at baseline Circulating Progenitor Cells and Coronary Microvascular Dysfunction: Results from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation - Coronary Vascular Dysfunction (WISE-CVD) Study Patients: • 160 women enrolled in the WISE-CVD Study with ischemia during stress testing • No obstructive CAD Protocol: • CFR measured as ratio of hyperemic average peak velocity (APV) in response to intracoronary adenosine to baseline APV • Lower CFR with adenosine correlated significantly with higher levels of CD34+, CD34+/CD133+ and CD34+/CXCR4+ cells Circulating Progenitor Cells and Coronary Microvascular Dysfunction: Results from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation - Coronary Vascular Dysfunction (WISE-CVD) Study Patients: • 160 women enrolled in the WISE-CVD Study with ischemia during stress testing • No obstructive CAD Protocol: • Microvascular endothelial function measured as the CBF response to intracoronary acetylcholine (n=48) • Lower CBF with acetylcholine correlated significantly with higher CD34+/CXCR4+ and CD34+/VEGF+. Progenitor Cells and ischemic syndromes • ACS is associated with mobilization of VEGF2R and CXCR4 expressing PCs • Subjects with microvascular coronary ischemia with reduced flow reserve and endothelial dysfunction have higher circulating PC subsets • PC expressing CXCR4, denoting cells with a capacity to home to areas of ischemia, are increased in those who develop ischemia during MS. • This suggests that even mild ischemia during daily living may stimulate PC mobilization. Circulating progenitor cells CD34+ cell populations Circulating progenitor cells and sub-clinical vascular disease Circulating progenitor cells and ischemic syndromes Circulating progenitor cells and CVD outcomes CD34+ cells as therapy for vascular diseases Risk factors, Vascular Injury, and Regenerative Capacity Risk Factors Endothelial injury Progenitor Cells Endothelial dysfunction, Arterial stiffness Atherogenesis Vascular Repair Relationship Between circulating Progenitor Cell Counts and Long term CVD outcomes (Death/MI) • • • • Patients undergoing coronary angiography: Treated with guideline based therapies 502 patients in a Discovery cohort ; 403 patients in a Validation cohort. Total Pooled 905; age 63yrs; 65% male • PCs were enumerated by flow cytometry as CD45med+ blood mononuclear cells expressing CD34, CD133, VEGFR2 and/or CXCR4 • Followed patients in each cohort for a mean of 2.7 & 1.2 years, for the primary endpoint of death or myocardial infarction (MI). 92 death/MI (10%) Higher counts of CD34+ and CD34+/CD133+ cells correlated with: younger age (p<0.001 both), male gender (p=0.04 and p<0.011) higher GFR (p<0.001 both) Higher CD34+/CD133+ also with greater BMI (p<0.001). 37 CD34+ Relationship Between circulating Progenitor Cell Counts and Event Free Survival (Major Events - Death/MI) C statistic improved from 0.713 to 0.752, p=0.024 for CD34/133+38 Relationship Between circulating Progenitor Cell Counts and Long term CVD outcomes (Death/MI) • • • • • • Patients undergoing coronary angiography 502 patients in a Discovery cohort ; 403 patients in a Validation cohort. Total Pooled 905; age 63yrs; 65% male PCs were enumerated by flow cytometry as CD45med+ blood mononuclear cells expressing CD34, CD133, VEGFR2 and/or CXCR4 Followed patients in each cohort for a mean of 2.7 & 1.2 years, for the primary endpoint of death or myocardial infarction (MI). 92 death/MI Cell Population CD34+ CD34+/133+ Outcome Low v High ROC Death/MI 2.76 (1.65-4.61) Death 2.60 (1.45-4.66) CV Death 2.35 (1.28-4.35) Death/MI 2.98 (1.68-5.30) Death 2.66 (1.40-5.03) CV Death 2.49 (1.28-4.86) CD34+/VEGF+ Death/MI 1.01(0.60-1.69) Death 1.18 (0.65-2.14) CD34+/133+/VEGF CV Death 1.34 (0.71-2.50) Death/MI 0.97 (0.59-1.59) Death 0.83 (0.47-1.47) CD34+/CXCR4+ CV Death 0.83(0.45-1.50) Death/MI 2.50 (1.19-5.23) Death 2.15 (1.00-4.62) CV death 1.82(0.82-3.96) 39 Relationship Between circulating Progenitor Cell Counts and Event Free Survival (Major Events - Death/MI) 40 Relationship Between circulating Progenitor Cell Counts and Long term CVD outcomes (Death/MI) Conclusion: Low levels of circulating PCs, defined as co-expression of CD34 and CD133 and CXCR4 epitopes are robustly associated with risk of future death/MI in patients with CAD. Implications; (1) CD34+/CD133+ cells that are enriched for bone marrow-derived hematopoietic and endothelial progenitors, may represent an index of global regenerative potential (2) PCs protect or regenerate damaged endothelium by local or systemic paracrine effects, (3) The predictive value of PCs as risk markers was equal or greater than conventional risk factors such as smoking. 41 Circulating progenitor cells CD34+ cell populations Circulating progenitor cells and gender Circulating progenitor cells and ischemic syndromes Circulating progenitor cells and CVD outcomes CD34+ cells as therapy for vascular diseases Atherosclerosis Incidence Risk PCs Repair Age or Risk Factor Years PCs Repair Risk Acknowledgements Emory Clinical Cardiovascular Research institute Arshed Quyyumi Viola Vaccarino Riyaz Patel Danny Eapen Nima Ghassemzadeh Ronnie Ramadan Ibhar Al Mheid Girum Mekonnen Joseph Poole Robert Neuman Pankaj Manocha Hatem Kassem Alanna Morris Ayaz Rahman Saurabh Dhawan Salman Sher Ying Liu Nino Kavtaradze Elizabeth Rocco Sherri Mcdonald Cath Lab Attendings and Staff Fellows/ Research Volunteers Emir Veledar A Maziar Zafari Laurence Sperling Edmund Waller Qunna Li DeCode Genetics Dean Jones PhD (Metabolomics) Charles Searles (miRNA) Greg Gibson PhD (Transcriptomics)