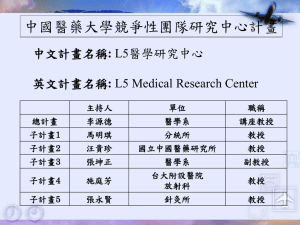
Stem and Progenitor Cell Therapy in Peripheral
Arterial Disease
Current knowledge and controversies
Drs. M. Teraa, MD 1,2
Prof. dr. M.C. Verhaar, MD PhD 2
Prof. dr. F.L. Moll, MD PhD 1
1
2
Department of Vascular Surgery, University Medical Center Utrecht, The Netherlands
Department of Nephrology and Hypertension, University Medical Center Utrecht, The
Netherlands
Backgrounds Critical Limb Ischemia (CLI)
•
Rest pain or tissue necrosis
•
500-1000 individuals per million persons per year
•
Important functional implications
•
Major impact on quality of life:
• Worse than in patients with terminal cancer, chronic
kidney or heart disease
•
CLI is associated with significant morbidity and mortality:
• 50% one year amputation free survival
Backgrounds CLI
•
~40% of the patients not eligible for surgical or endovascular
revascularization:
• Anatomic location of the lesion
• Extent of disease
• Extensive co-morbidity
•
No effective pharmacological therapy
•
Amputation often remaining option
New revascularization strategies needed
Cell therapy is a promising option
Origin of the endothelium
Backgrounds cell therapy
EPCs and therapeutic implications
Endothelial
Progenitor
Cells (EPC)
Neovascularisation
after ischemia
Asahara, 1997
Glomerular
Capillary Repair
Rookmaaker, 2002
Pancreas
Regeneration
Gloerich, 2004
• Limb salvage after
injection of human
EPC
Gene and growth factor therapy
• Pilot studies have shown promising results for:
• Vascular Endothelial Growth Factor (VEGF)
• Hepatocyte Growth Factor (HGF)
• Fibroblast Growth Factor (FGF)
• Hypoxia Inducible Factor-1α (HIF-1α)
• Larger trials have shown rather disappointing results
What about cell therapy?
Cell therapy – TACT Study (2002)
First Clinical trial
•
Pilot study (25 patients)
• BM-MNC vs saline
• Significantly improved ABI, TcPO2, rest pain,
pain-free walking time at 4 and 24 weeks
•
Randomized trial (22 patients)
• BM-MNC vs PB-MNC
• Significant improvement for BM-MNC
Adapted from Tateishi-Yuyama et al. Lancet. 2002;360(9331):427-35
Current results on cell therapy in CLI
Adapted from Fadini et al. Atherosclerosis. 2010;209(1):10-17
Need for confirmation!!!
• Large randomized controlled trials
• More clinical relevant end points:
• Amputation
• Mortality
• Quality of Life
• Elucidate the black box of cell therapy
Black Box
Patient characteristics?
Administration route?
Cell type?
Cell source?
Mechanism?
Which patient will benefit?
• Young vs. Old
• Diabetics vs. Non-diabetics
• Male vs. Female
• Chronic kidney disease
• BMI
• Smokers vs. Non-smokers
• Fontaine III vs. Fontaine IV
• Buerger’s disease vs. Atherosclerosis obliterans
The cell?
• Clinically studied:
• Mobilized PB-MNCs
• BM-MNCs
• CD34+ selected cells
• BM-MSCs (Bone marrow derived mesenchymal stem cells)
Adapted from Fadini et al. Atherosclerosis. 2010;209(1):10-17
The way?
• Intra-arterially or intra-muscular
• Combined intra-arterial and intra-muscular
• Once or repetitive administration
• Amount of cells needed:
• Feasibility versus effectiveness
Future perspectives for cell and source
•
Cell sources:
• Blood
• Bone marrow
• Fat
• Cord blood
• Embryonic
• iPSCs (induced Pluripotent Stem Cells)
•
Cell types:
• MNC
• MSC
• Cultured EPC:
•
Endothelial Colony Forming Cells (ECFCs)
•
Circulating Angiogenic Cells (CACs)
Lu et al. Diabetes Res Clin Pract. 2011; [Epub ahead of print]
Future perspectives in cell therapy
• Progenitor cell dysfunction in cardiovascular diseases
• Pretreatment of cells could -partly- restore dysfunction:
• Statins
• Antioxidants
• NO-donors
• PPAR-γ agonists
Adapted from Sasaki et al. Proc Natl Acad Sci USA. 2006; 103(39): 14537-41
Harvesting methods
• Marrow miner:
• Claims to harvest more progenitor cells
• Commercial kits? Be aware:
• GMP-guidelines
• Aseptic isolation and culture
• Knowledge of the product
• Efficacy still not established
Clinical trials are still the way to go!!!
Large trials
• 21 ongoing trials studying stem/bone marrow cell therapy in
PAD
• Significant commercial input
Lawall et al. Thromb Haemost. 2010; 103(4): 696-709
JUVENTAS* Trial (NCT00371371)
* ReJUVenating ENdothelial
bone marrow
(100 ml)
BM-MNC
Infusion
progenitor cells via Transcutaneous
intra-Arterial Supplementation
versus
bone marrow
(100 ml)
Placebo
Storage
Juventas-trial
Design
-
Randomized, placebo-controlled, double-blinded
clinical trial
Highlights
•
Started in September
2006
•
Design with repeated
intra-arterial infusion
•
Over 20 referring
centers
•
Group sequential
interim analyses for
safety and efficacy
•
Coupled basic
research
Objectives
-
Evaluate the effects of repeated intra-arterial
infusion of BM-MNC in 110 – 160 CLI patients
-
Study functional characteristics of BM-MNC and
relate dysfunction to clinical outcome
Currently 100 patients included!!!
Juventas Study Group
Steering Group
Drs. M. Teraa
Dr. R.W. Sprengers
Prof. Dr. M.C. Verhaar
Prof. Dr. F.L. Moll
Dr. R.E.G. Schutgens
Dr. I.C.M. Slaper-Cortenbach
Prof. Dr. Y. van der Graaf
Prof. Dr. W.P.Th.M. Mali
Prof. Dr. P.A. Doevendans
Dr. A. de Wit
For more information:
www.juventas-trial.nl
Data Safety Monitoring Committee
Prof. Dr. A. Algra
Dr. I. van der Tweel
Prof. Dr. T.J. Rabelink
The JUVENTAS trial is made
possible by grants of:
Foundation ‘De Drie Lichten’
Question 1
• From an embryonic point of view endothelial progenitor cells are
closest related to:
a)
Neural cells
b)
Stromal cells
c)
Haematopoietic cells
d)
Adipocytes
e)
Hepatocytes
Answer question 1
• From an embryonic point of view endothelial progenitor cells are
closest related to haematopoietic cells (answer C).
Both the primitive vasculature and haematopoietic cells develop
from the blood ilands as a common origin. Endothelial progenitor
cells do express both markers expressed on haematopoietic and
endothelial cells further underlining their similar ontologic relation.
Question 2
• The most appropriate way to administer progenitor cells in
patients with peripheral arterial disease is:
a) Intramuscular
b) Intraarterially
c) Combination of A and B
d) Still unclear
Answer question 2
•
The most appropriate way to administer progenitor cells in patients with peripheral arterial disease is still unclear
(answer D)
Most studies conducted so far have studied the intramuscular method to apply cell based therapies. A smaller amount
of studies performed intra-arterial infusion. Just one study analyzed a combination of both administration routes. Based
on current evidence there seems no clear difference of the administration route applied. Intramuscular administration
seemed to perform somewhat better, this was not significant however and needs to be confirmed in controlled studies.
Fadini, Agostini, Avagaro. Atherosclerosis 2010; 209: 10-17
Question 3
• Based on current literature clinical effects of cell therapy is not
known to be influenced by:
a) Cell source
b) Number of cells infused
c) Underlying cause of limb ischemia
d) Sex
Answer question 3
•
Based on current literature clinical effects of cell therapy is not known to be influenced by sex (answer D).
Clinical effects (ABI, tcPO2) have been shown to be somewhat better in bone marrow derived cells and probably
even better in bone marrow derived mesenchymal stem cells. Effects of cell based therapies have almost
unequivocally been better with increasing amount of cells administered (mainly number of CD34+-cells).
Moreover, ther underlying condition causing the critical limb ischemia clearly plays a role in the effects observed
after cell therapy. Patients suffering from atherosclerosis obliterans have shown to be respond better than
patients with thromboangitis obliterans. Sex has not yet been shown to influence effects observed after cell
therapy.
Lu, Chen, Liang et al. Diabetes Res Clin Pract 2011; Epub ahead of print
Fadini, Agostini, Avagaro. Atherosclerosis 2010; 209: 10-17
Idei, Soga, Hata et al. Circ Cardiovasc Interv. 2011; 4: 15-25
Question 4
• What’s true about cell based therapies in peripheral arterial
disease:
a) Cell therapy is widely accepted as a conventional therapy
b) Its effects on clinical relevant end points still need to be
confirmed
c) Malignant transformation is a serious and common
reported problem in cell therapy
d) Growth factor therapies seem equally effective
Answer question 4
•
What’s true about cell based therapies in peripheral arterial disease: “Its effects on clinical relevant end
points still need to be confirmed.” (answer B)
Cell based therapies have generally shown to improve surrogate end points, such as ABI and tcPO2. Pain free
walking distance, ulcer healing and amputation-free survival have also been reported to improve. Most studies
conducted were small non-controlled studies and therefore not designed or underpowered to draw definitive
conclusions on clinical relevant end points.
Fadini, Agostini, Avagaro. Atherosclerosis 2010; 209: 10-17
Sprengers, Moll, Verhaar. Eur J Vasc Endovasc Surg 2010; 39: S38-43