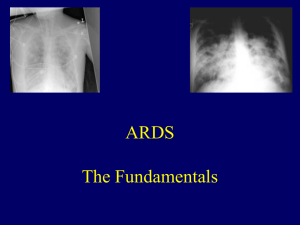
The Acute Respiratory Distress Syndrome: A review of the literature
advertisement

The Acute Respiratory Distress Syndrome: An update of the current literature Judson Mehl, DO Tulane University School of Medicine Department of Anesthesiology Key Points History of acute respiratory distress syndrome (ARDS) in adults Past and current definitions Incidence of ARDS Risk factors for ARDS Current understanding of pathophysiology Interventions – what has worked, and what has not Ongoing research What is ARDS? A common and life-threatening condition May be lone insult or complication of: Critical Illness Sepsis Pneumonia Trauma Other Lung inflammation, micro and macroatelectasis, hypoxemia Ventilator dyssynchrony, frequent barotrauma History: First recognized as a clinical syndrome in 1967 Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute Respiratory Distress in Adults. Lancet. 1967; 2(7511):319-323 Rapidly progressive respiratory failure Noncardiogenic pulmonary edema Severe arterial hypoxemia Requiring mechanical ventilation 1994 American-European Consensus Conference Simplified definitions Current treatment strategies Future research 1994 AECC Consensus Definition: Definitions based on PaO2/FiO2 ratio ALI vs. ARDS Absence of left atrial hypertension PEEP requirements not considered in stratification AECC AECC definition has been widely adopted Allowed for clinical and epidemiologic data gathering on ARDS and ALI Has led to improved outcomes and better care But it has limitations: Timing – “acute” undefined ALI – confusing terminology Oxygenation – does not account for PEEP Radiograph criteria – unclear; poor intraobserver reliability PAWP – High PAWP and ARDS may coexist; The Berlin Definition JAMA June 2012 Commissioned by the European Society of Intensive Care Medicine Endorsed by the American Thoracic Society and Society of Critical Care Medicine Assess the predictive value of ancillary variables using empirical data Refine the definition Starting Point: Conceptual model: Acute, diffuse inflammatory injury Increased vascular permeability Increased lung weight Loss of aerated lung tissue Clinical hallmarks: Hypoxemia Bilateral chest radiograph opacities Increased venous admixture Increased dead space Decreased lung compliance Consensus proposed changes: Definition: 3 mutually exclusive categories: Mild, Moderate, Severe Ancillary variables to characterize “severe” Further empirical evaluation of these variables Timing – Symptoms within one week of known clinical insult or worsening respiratory symptoms Chest Imaging – Retained definition of bilateral opacities Proposed “Severe” variable for emperical evaluation: More extensive opacity (3 or 4 quadrants of the radiograph) Consensus proposed changes: Pulmonary edema: PAWP criteria removed from the definition Patient meets ARDS criteria if they have respiratory failure not fully explained by cardiac failure or volume overload Oxygenation: Remove ALI from the definition Proposed “Severe” variable for emperical evaluation: Minimum PEEP level of 10 cmH20 Consensus proposed changes: Additional Measurements: Minute Ventilation standardized to a PaC02 of 40 mmHg Surrogate measure for lung dead space, increased mortality VECORR = ( minute ventilation X (PaCO2)/40 Respiratory System Compliance (< 40mL/cm H20) ) Cohort Assembly Thorough review of the literature presented at consensus meeting Study eligibility criteria: 1. Large, multicenter prospective cohorts or smaller, single-center prospective studies with unique radiologic or physiologic data which enrolled patients meeting the AECC definition of both ARDS and ALI Cohort Assembly Thorough review of the literature presented at consensus meeting Study eligibility criteria: 2. Data collection sufficient to apply the individual criteria of both the Berlin Definition and the AECC Definition 3. Authors of the studies willing to share data and collaborate 7 Distinct data sets identified with sufficient information 4 multi-center clinical studies (Clinical database) 3 single-center physiologic studies (Physiologic database) 4188 Patients Variables 90-day mortality Ventilator-free days at 28 days following the diagnosis of ALI Duration of mechanical ventilation Progression between stages 4 Ancillary Variables “Severe ARDS” PaO2/FIO2 ratio of 100 or less 3 or 4 quadrant opacities on radiograph PEEP 10cmH20 or higher CRS less than 40 mL/cm/H2O or . . . VECORR greater than 10 L/min Would these variables identify a group of patients with higher mortality than the high risk group simplified to : PaO2/FiO2 <100 ? P<.001 comparing mortality across stages of ARDS for draft and final definitions However, Because the Berlin Definition has just been introduced into clinical practice . . . The literature which follows will still utilize the nomenclature ALI vs. ARDS under the previously stated AECC definitions. Incidence Annual incidence ranges 140,000 to 190,000 cases per year in the US adult population Mortality rate ranges between 26-58% Rubenfeld DG, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005; 353 (16): 1685-1693 Across the World: Risk Factors for ARDS Gastric Aspiration Sepsis Trauma Multiple blood transfusions Many others suggested Still being studied Risk Factors Risk factors for increased mortality From multicenter epidemiologic cohorts: Older age Worse severity of illness Shock on hospital admission Increased radiographic opacity Immunosuppression Emerging research Role of chronic alcohol abuse Role of genetics Role of environmental factors Treatment strategies Chronic Alcohol Review of several previous articles: Alcoholics more likely to suffer from: trauma pneumonia gastric aspiration sepsis pancreatitis Alcohol is an independent risk factor for development of ARDS Guidot DM, Hart CM. Alcohol abuse and acute lung injury: epidemiology and pathophysiology of a recently recognized association. J Investigative Med. 2005; 53: 235-245 Alcohol increases risk for multiorgan dysfunction in association with ARDS Animal Model Rats fed 20% ethanol in drinking water for 5 weeks In ex-vivo lung preparation, ethanol exposed rats had more edema than control rats after induced endotoxemia Type II alveolar epithelial cells from ethanol-exposed rats had decreased ability to synthesize and secrete surfactant Guidot DM, et al. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol. 2000 Jul;279(1):L127-35. More susceptible to oxidantinduced cell death when exposed to hydrogen peroxide Animal Model Alveolar epithelial permeability to radiolabeled albumin was 5x greater in isloates from ethanol-fed rats than the control Alveolar epithelium from ethanol-fed rats had increased expression of apical sodium channels Counteract increased paracellular leak Maintains balance in the absence of further oxidative stress These compensatory mechanisms are overwhelmed in the face of an inflammatory challenge Result is proteinaceous fluid leak The role of Glutathione The role of glutathione depletion in alcohol-induced hepatic injury is well established The concept of glutathione depletion in lung tissue is novel Several animal studies demonstrate that ethanol ingestion decreases glutathione levels by 80% in epithelial lung fluid 90% in lung epithelial cells Subsequent studies demonstrate that supplementation of glutathione in the experimental diet prevents ethanol-mediated defects in lung epithelium. Human Correlate When compared with non-alcoholic controls: Otherwise healthy alcoholic subjects have dramatically decreased levels of glutathione in lung lavage fluid These decreases correlate proportionally with that seen in the animal model Moss M, Guidot DM, Wong-Lambertina M, et al. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med 2000; 161:414-9 To be continued . . . Studies on glutathione supplementation in alcoholics with ARDS are ongoing Genetics As with any disease, the genetics of ARDS are complicated Over 25 separate genes have been identified and studied in regards to clinical outcome These genes tend to regulate Inflammation Coagulation Endothelial cell function Reactive oxygen species generation Apoptosis FAS Genetic Variation FAS ligand binds to FAS receptor on cell surface Cascade of inflammation and apoptosis Increased levels of FAS ligand found in BAL fluid in previous studies Are genetic polymorphisms of FAS associated with development of ALI? FAS Genetic Variation 14 FAS polymorphisms evaluated Healthy controls vs. FACTT patients 3 polymorphisms identified. These halotypes had higher levels of blood FAS mRNA and increased mortality vs. controls Other inflammatory pathways also involved: FAS is not alone. Other studies have identified associations with ALI/ARDS with deregulated inflammation in other pathways: NFKBIA (Nuclear Factor of Kappa Light Chain Enhancer in B-Cell Inhibitor) LTA (lymphotoxin alpha) MYLK (myosin light chain kinase) ACE (angiotensin conversion enzyme) NAMPT (Nicotinamide Phosphoribosyltransferase) Associations shown with mortality, duration of mechanical ventilation Other polymorphisms currently under review: T-46C polymorphism in the promoter region of Duffy Antigen chemokine receptor (DARC) Associated with a 17% increase in mortality, specifically in African American patients in ARDS-Network clinical trials And others: PPFIA1 shown to increase susceptibility for ALI after major trauma Polymorphisms in other receptors showed worse outcomes with specific infectious agents: pneumococci, Legionella, virus Key point: Genetics of both the host and the microbe are likely both highly important in the degree of inflammation and subsequent development of ARDS Pathogenesis Central concepts: Dysregulated inflammation Uncontrolled activation of coagulation pathways Inappropriate accumulation of leukocytes, platelets Disrupted alveolar endothelial barriers Inflammatory mechanisms necessary for pathogen clearance Controlled vs. excessive Leukocyte protease release Generation of reactive oxygen species Abundant synthesis of chemokines, cytokines Toll-like receptor engagement Toll-Like receptor: A major player Pattern-recognition receptor “Pathogen-associated molecular patterns” Single-spanning non-catalytic receptor molecule Highly expressed on macrophages and dendritic cells Innate immune response activation Work in tandem with Interleukin-1 receptor Vascular endothelial Cadherin Adherens protein critical for integrity of endothelial barrier in lung microvasculature Bonds between proteins Bonds destabilized by TNF, Thrombin, VEGF, leukocyte signaling molecules and even anti-VE-Cadherin-Ab Experimental manipulation of the stability of VE-Cadherin bonds alter the leukocyte transmigration through cellular junctions In LPS challenged mice, stabilization of these bonds decreased BAL protein contend and leukocyte content What do the clinical trials show? Research focused on: Lung-protective ventilation High PEEP Prone positioning NMBD Steroids Fluid conservative vs. liberal ECMO Also, APC, GM-CSF, inhaled beta agonists, nitric, omega-3 FA none of which have showed mortality difference I will present the largest and most thorough of the trials currently published Again, research relating to treatment of ARDS is a very active and ongoing field Lung-protective ventilation 861 patients enrolled Randomized to 12ml/kg predicted body weight Plateau pressures up to 50 6ml/kg predicted body weight Plateau pressures up to 30 Primary outcomes: Mortality prior to discharge ARDS Definition Task Force. JAMA 2012;307:2526 -2533 Secondary outcomes: Ventilator-free days through hospital day 1-28 Enrollment Patients admitted to ARDS-NET hospitals PaO2:FiO2 ratio of 300 or less Bilateral pulmonary infiltrates No evidence of LA hypertension PCWP less than 18mmHg (if measured) Exclusion criteria: >36 hours since meeting criteria Pregnant Less than 18 y.o. Burns Increased ICP Other conditions with estimated 6-month mortality>50% Other LPV trials Amato MB, et al. Effects of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. NEJM 1998; 338: 347-354 N=53 Decreased mortality Villar J, Kacmarek RM, Perez L. A high positive end-expiratory pressure, low tidal volume ventilator strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Critical Care Medicine 2006; 34:1311-1318 N=103 Decreased mortality High PEEP trials 549 patients Meeting ARDS criteria Randomized to high vs. low PEEP Brower RG, et al. Higher versus lower positive end-expiratory pressure in patients with the acute respiratory distress syndrome. NEJM. 2004; 289:2104-2112 Ventilator settings per protocol Mean age in the higher PEEP group was significantly higher PaO2:FiO2 in high PEEP group was significantly lower Protocol change after 170 patients enrolled Trial stopped after 549 patients based on predetermined futility stopping rule No significant difference between the two groups in : Mortality ICU days Ventilator-free days Organ-failure free days High PEEP trials Meade MO, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers and high positive endexpiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008; 299:637-645 N=385 No mortality difference. No difference with recruitment maneuvers Mercat A, et al. Positive end-expiratory pressure setting in adults with aculte lung injury and acutre respiratory distress syndrome: a randomized controled trial. JAMA. 2008; 299: 646-655 N=382 No mortality difference But for the sake of argument . . . Meta analysis of previous trials N=2299 patients with ARDS or ALI No difference in hospital mortality overall Small statistical difference in high PEEP group, specifically in subset with ARDS Prone Position Multicenter RCT 342 patients Stratified into moderate vs. severe based on PaO2:FIO2 ratio Primary Outcome: 28 day mortality Taccone P, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009; 302:1977-1984 Secondary: 6-month mortality Organ dysfunction Complication rate from prone positioning Prone Position Patients randomized into prone vs. supine Prone patients remained prone for 20 hours/day until Resolution of ARDS or End of 28-day study period Tidal volumes limited to 8cc/kg with max plateau pressures of 30 mmHg Conclusions Prolonged prone position not associated with survival advantage No detectable difference in : 28 day mortality 6-month mortality Ventilator free days ICU length of stay The incidence of many of the studied complications were higher in the prone group NMBD Meta-analysis of 3 RCT N= 431 patients 48-hour infusions of cisatracurium in patients with ARDS Alhazzanui W, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Critical Care. 2013;17 Outcomes: Barotrauma Duration of ventilation ICU-acquired weakness NMB Several previous small trials #1 – improved oxygenation with continuous cisatracurium infusion #2 – significant reduction in inflammatory mediators in blood and BAL fluid with patients on cisatracurium #3 – no difference in crude hospital mortality rates Findings: Cisatracurium infusion for 48 hours: Reduced risk of death at 28 days Reduced risk of death at ICU discharge Reduced risk of death at hospital discharge Reduced the risk of barotrauma No effect on the the duration of mechanical ventilation No effect on the risk of ICU weakness These findings were strongly significant in terms of mortality reduction Methylprednisolone Trial looking at persistent ARDS Defined – ARDS of at least 7 days duration N= 180 patients Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009; 302:1977-1984 Randomized to placebo or solumedrol Methylprednisolone Primary – mortality at 60 days Secondary – Ventilator-free days Organ-failure free days Inflammatory mediator levels Infectious complications Protocol – 2mg/kg bolus, then 0.5mg/kg Q6 hours for 14 days, then 0.5 mg/kg Q 12 hours for 7 days Conclusions No beneficial effect on hospital mortality rate Starting steroids 2 weeks or more after onset of ARDS increased mortality at 60 and 180 days Conclusions Steroids did improve cardiopulmonary physiology variables between days 3 and 7 Steroids increased the number of ventilator-free days, ICU-free days at day 28 Steroid patients were able to breathe without assistance earlier, but were more likely to require resuming of assisted ventilation There was no difference in length of hospitalization between the two groups Other Steroid Trials Bernard GR, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. NEJM. 1987; 317:1565-1570 No mortality difference Meduri GU, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998; 280:159-165 Small mortality decrease Meduri GU, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007: 131: 954-963 Slight reduction in duration of mechanical ventilation. No mortality difference The BIG picture: ARDS is a complicated and multi-factoral process Likely genetic and environmental components Treatment strategies Lung protective ventilation – HELPFUL High PEEP – Likely not helpful, though maybe small benefit in ICU death rate in patients with ARDS Prone – Likely not helpful,Possibly harmful NMBD – Probably helpful Steroids – Likely not helpful Fluid conservative therapy – Possibly helpful Citations: 1. Matthay M, Ware L, Zimmerman G. The acute respiratory distress syndrome. J Clin Invest. 2012; 122:2731-2740 2. Rubenfeld MD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest. 2007; 131:554-562 3. ARDS Definition Task Force. JAMA 2012;307:2526 -2533 4.Guidot DM, Hart CM. Alcohol abuse and acute lung injury: epidemiology and pathophysiology of a recently recognized association. J Investigative Med. 2005; 53: 235-245 5. Amato MB, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. NEJM. 1998;338: 347-354 6. [No Author Listed]. Ventilation with lower tidal columes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. ARDS Network. NEJM. 2000; 342:1301-1308 7. Brower RG, et al. Higher versus lower positive end-expiratory pressure in patients with the acute respiratory distress syndrome. NEJM. 2004; 289:2104-2112 8.Curley MA, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005; 294:229-237 9. Taccone P, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009; 302:1977-1984 10. Papazian L, et al. Neuromuscular blockers in early acute respiratory distress syndrome. NEJM. 2010; 363:1107-1116 11.Alhazzanui W, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and metaanalysis of randomized controlled trials. Critical Care. 2013;17 12. Steinberg KP, et al. Efficacy and safety of corticosteroids for persistent acure respiratory distress syndrome. NEJM. 2006; 354:1671-1684 13. Meduri GU, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280:159-165 14. Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. NEJM. 2006;354:2564-2575 15. Shariatpanahi ZV, et al. Effect of enteral feeding with ginger extract in acute respiratory distress syndrome. J of Crit Care. 2012.