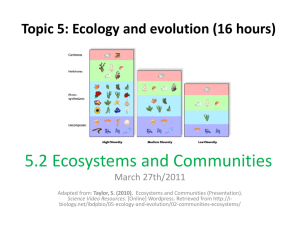
trophic level - Plain Local Schools
advertisement

36.1 Feeding relationships determine the path of energy and chemicals in the ecosystem I. Energy Flow and Chemical Cycling A. Energy enters an ecosystem as light. B. Photosynthetic producers, like plants, change light energy to chemical energy (organic compounds). C. Consumers obtain chemical energy by feeding on producers or on other consumers. D. Decomposers break down wastes and dead organisms. E. As living things use chemical energy, they release heat/thermal energy. F. Energy is not recycled within an ecosystem, but flows through it and out. (light energy into chemical energy into heat energy) G. Chemicals, such as C, O, & N, can be recycled between living & nonliving parts of ecosystems & the biosphere II. Food Chains A. A food chain is a pathway of food transfer from one trophic level (feeding level) to another (see Figure 36-2). B. Producers make up the trophic level that supports all others. C. Consumers are the organisms in trophic levels above producers. D. Consumers can be categorized by what they eat: 1. Herbivores eat only producers. (i.e. cow eats grass) 2. Carnivores eat only other consumers. (i.e. lion eats zebra) 3. Omnivores eat both consumers and producers. (i.e. bear eats salmon & berries) E. Consumers can be categorized by position in a food chain. 1. Primary consumers feed directly on producers (i.e.grasshoppers eating plants) 2. Secondary consumers eat primary consumers. (i.e. mice eating grasshoppers); Tertiary eat secondary… F. Decomposers feed on & break down detritus (wastes & remains of dead organisms). 1. The main decomposers are bacteria and fungi; they’re abundant in soil. 2. All ecosystems include decomposers even though most food chains don’t show decomposers. III. Food Webs A. Feeding relationships are usually more complicated than shown in simple food chains. B. Ecosystems contain many different species that have a variety of food sources. C. Food webs show the feeding relationships between interconnected & branching food chains A Desert Food Web 36.2 Energy flows through ecosystems I. Productivity of Ecosystems A. Available energy or energy budget is limited in an ecosystem. B. For most ecosystems, the amount of sunlight that enters the ecosystem determines the budget. C. Earth’s producers make billions of kilograms of organic material, or biomass, each year. Productivity D. The rate at which producers build biomass is called primary productivity. E. Primary productivity determines the maximum amount of energy available to all the higher trophic levels in an ecosystem. Productivity F. Productivity is different for different ecosystems: 1. Tropical rainforests have the highest productivity. Why? Their warm moist climate supports year-round growing. 2. Productivity is low in typically dry & cold Tundra. 3. Conditions are more moderate for producers in grasslands Productivity Rain Forest Grasslands Tundra Productivity G. Exception – organisms living in deep dark oceans do not get their energy from the sun; prokaryotic producers can extract energy from sulfur compounds released by hydrothermal vents to make organic compounds. II. Ecological Pyramids A. Energy is “spent” at each step of the food web. B. As each consumer feeds, some energy is transferred from the lower trophic level to the higher trophic level. C. An average of 10% of the available energy at a trophic level is converted to biomass in the next higher trophic level. D. The rest of the energy (about 90%) is lost as heat. E. The amount of energy available to top-level consumers is tiny compared to that available to primary consumers. F. It takes a lot of vegetation to support higher trophic levels. G. Most food chains are limited to three or four levels because there is not enough energy at the top of the energy pyramid to support another trophic level. H. Ecological pyramids are diagrams used to depict information about energy, biomass, and numbers of organisms at different trophic levels. An Energy Pyramid 36.3 Chemicals cycle in ecosystems I. The Basic Pattern of Chemical Cycling A. Chemical cycles usually involve three general steps: 1. Producers incorporate chemicals from the nonliving environment into organic molecules. 2. Consumers feed on the producers, incorporating some of the chemicals into their own bodies and releasing some back into the environment as waste. 3. Organisms die & decomposers break them down, supplying soil, water, & air with chemicals in inorganic form. The producers gain a renewed supply of raw materials for building organic matter, & the cycles continue. B. Part of each chemical’s cycle involves nonliving processes like rain and fires. II. The Carbon and Oxygen Cycle A. Carbon is found in inorganic form in the atmosphere as CO2 gas and dissolved in water as HCO3- . B. Producers use the carbon and oxygen atoms to form organic compounds during photosynthesis. C. Some organic compounds cycle to consumers as food. Carbon-Oxygen Cycle D. During cellular respiration producers & consumers break down organic compounds and release CO2 gas as a waste product. E. Decomposers break down detritus and release CO2 gas. F. Burning fossil fuels (coal, oil, natural gas) & wood releases CO2 gas into the atmosphere. G. Volcanic eruptions add CO2 gas to atmosphere. Carbon-Oxygen Cycle Forms organic compounds during photosynthesis During cellular respiration both consumers and producers release carbon dioxide Non-living processes – burning fossil fuels, forest fires and human activities also release carbon dioxide into the atmosphere Decomposers break down detrius – releasing carbon dioxide III. The Nitrogen Cycle A. Nitrogen is found in all living organisms in amino acids & other essential molecules. B. Almost 78% of Earth’s atmosphere is nitrogen gas (N2). C. Most producers can only use nitrogen in the form of compounds like ammonium (NH4+) and nitrate (NO3-). D. Nitrogen-fixing bacteria convert N2 gas to ammonia (NH3) in a process called nitrogen fixation. Nitrogen Cycle E. Nitrogen-fixing bacteria live in soil and in nodules on roots of plants (peas, beans, alfalfa). F. Other bacteria in soil convert NH4+ to nitrates in a process called nitrification. G. Producers absorb ammonium and nitrates and use them to build amino acids, proteins, and nucleic acids. Nitrogen Cycle H. Consumers that eat producers obtain nitrogen. I. Decomposers release nitrogen (ammonium) from wastes and decaying organisms. J. Denitrifying bacteria in soil convert some nitrates to N2 gas back into atmosphere. Nitrogen Cycle Lives in soil and in nodules on roots Decomposers release nitrogen from wastes and decay (as ammonium) Producers absorb ammonium and nitrates from the soil and use them to build amino acids, proteins and nucleic acids. Consumers eat the producers – obtaining nitrogen Ammonia picks up another hydrogen ion from water to form ammonium Nitrification – other bacteria in the soil converts ammonium into nitrates IV. Water Cycle A. Sun’s energy evaporates water putting water vapor into atmosphere. B. Water vapor cools and condenses and falls as precipitation. C. Plants absorb fresh water from soil. D. Consumers obtain water from eating and drinking. E. Water evaporates from the leaves of plants = transpiration. F. Some water runs off land into rivers and streams and some restores groundwater. Water Cycle Falls as rain, snow, hail or sleet Evaporation from the leaves of plants 36.4 Human activities can alter ecosystems I. Human Activities Impact Carbon Cycle A. Deforestation – clearing forests for agriculture, lumber, etc., affects carbon cycle by eliminating the plants that remove CO2 from atmosphere. - burning the trees would increase CO2 and accounts for about 20% of CO2 added to atmosphere by human activity. B. Burning fossil fuels increases CO2 levels & accounts for about 80% of CO2 added to atmosphere by human activity. Impact the Carbon cycle C. Adding CO2 increases greenhouse effect (a natural process) 1.CO2 & water vapor are some heat absorbing gases. 2. These greenhouse gases let sunlight through but then trap heat radiated from Earth’s surface. 3. increasing them could possibly lead to global warming (see Fig. 36-14) Impact the Carbon cycle D. Global warming is an increased average temperature worldwide E. Possible effects of global warming (even by just a few degrees): 1. melting of glaciers & polar ice caps. 2. rise in sea level & flooding low-lying coastal areas. 3. changes in weather patterns (precipitation). 4. boundaries between biomes might shift and affect species. Carbon dioxide II. Human Activities Impact Nitrogen Cycle A. Some sewage treatment plants release dissolved nitrogen compounds into streams & rivers. B. Fertilizers can run off into streams & ponds. C. High levels of nitrogen (and phosphates) in the water can cause eutrophication – rapid growth of algae (algal bloom), which later die. Bacteria decompose algae and use up so much of the oxygen that the body of water can no longer support other organisms. Eutrophication Impact Nitrogen Cycle D. Burning fossil fuels releases nitrogen and sulfur compounds into air. These compounds combine with water in the air and form nitric and sulfuric acid. Precipitation with these acids is called acid rain. 1. Compounds in the atmosphere can travel great distances. Compounds produced in the Midwest of the U.S. create acid rain problems in Eastern Canada 2. Clean Air Act has lessened problem in U.S. by reducing levels of sulfur emissions. III. Human Activities Impact Water Cycle A. Deforestation of tropical rainforests greatly reduces amount of water vapor added to atmosphere by transpiration. - this can affect precipitation patterns which affects ecosystems. B. Drawing water from rivers and underground aquifers faster than it’s replaced could cause the aquifers to run dry. IV. Other Effects of Pollution A. Biological Magnification – the process by which pollutants become more concentrated in successive trophic levels of a food web - example: PCBs from industrial wastes were detected in tissues of organisms from the Great Lakes. PCB levels increased from 0.025 parts per million (ppm) in phytoplankton to 124 ppm in herring gull eggs. - example: DDT caused shells of eagle eggs to break easily. DDT use has been banned in the U.S. and population of eagles has recovered. Effects of Pollution B. The Ozone Shield, which absorbs harmful ultraviolet radiation, has been thinning since the 1970’s. Chlorofluorocarbons (CFCs) released from aerosol cans, refrigeration units, etc., destroy ozone (O3) molecules (see Fig. 36-18) 1. Increased exposure to UV radiation can cause: - increased rates of skin cancer & cataracts. - crop yields lessened & other producers harmed. 2. CFCs have been banned in many countries. 36.5 Conservation biology can slow the loss of biodiversity I. Why Diversity Matters A. Many of the species in an ecosystem are interconnected. -Ex. if one species disappears, other species & the health of the whole ecosystem may be affected. B. People value biodiversity (variety of life on Earth) because: 1. organisms & ecosystems are sources of beauty & inspiration. 2. organisms are sources of oxygen, food, clothing, & shelter. 3. 25% of all medicines contain substances that come from plants. C. It’s important to conserve biodiversity for future uses & needs. II. Threats to Biodiversity A. Throughout Earth’s history species have become extinct – the last member of the population died and the species no longer exists on Earth. B. Periods of mass extinction occurred as a result of dramatic climate changes from volcanic eruptions & asteroid impacts. (ex.Dinosaur extinction at end of Cretaceous period). Threats to diversity C. Currently a mass extinction is taking place on Earth. It’s scale is uncertain because the 1.5 million known species are only a fraction of the total on Earth. There are signs that species are disappearing at a dramatic rate (page 806). Threats to diversity D. What threatens biodiversity? 1. Pollution 2. Habitat Destruction – as human population grows, more land is cleared for agriculture, roads, and communities. 3. Introduced (non-native) Species often prey on native species & compete with them for resources. 4. Overexploitation – the practice of harvesting or hunting to such a degree that the small number of remaining individuals may not be able to sustain a population. III. Conservation Biology A. Focusing on Biodiversity Hot Spots, small geographic areas with high concentrations of species (see Fig. 36-23) 1. Many tend to be hot spots for extinction. 2. Global efforts are being taken to preserve some hot spot areas. B. Understanding an Organism’s Habitat – to manage existing habitat or to create a new one for a species Conservation biology C. Balancing Demands for Resources – efforts to save species often conflict with the economic & social needs of people. D. Planning for a Sustainable Future 1. Nations establish zoned reserves – areas of land that are relatively undisturbed by humans, surrounded by buffer zones which are minimally impacted by humans. 2. Sustainable development – developing natural resources so that they can renew themselves & be available for the future. (ex. Selectively harvesting timber)