Lignins in the ocean
advertisement
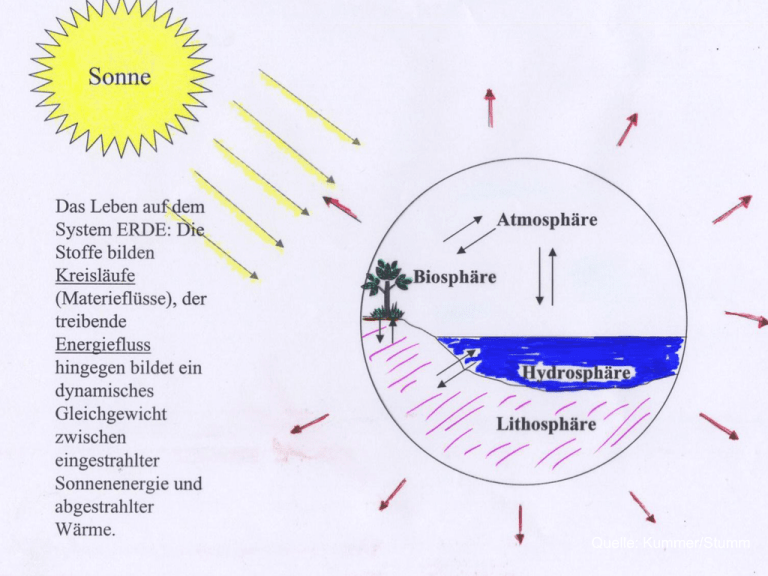
Quelle: Kummer/Stumm Ein wichtiges Konzept der Umweltchemie ist es, globale und regionale Materieflüsse und Stoffkreisläufe in den Vordergrund zu stellen Insbesondere die biogeochemischen Stoffkreisläufe der Elemente gut erforscht sind der globale Kohlenstoffkreislauf, Stickstoffkreislauf, Schwefelkreislauf u.a. Rolle natürlicher organischer Komplexbildner für Eisen The higher dissolution of Fe hydroxide in seawater has been attributed to the presence of organic ligands. (Liu and Millero, 2002; Hiemstra und Riemsdijk, 2006) Our research will contribute to deeper understanding of peatlands and their ecosystem services, thereby providing scientific basis for “ecosystem infrastructure” programs. (a) Net global peatland C sequestration rates per millennium (gigatons C per kiloyear (b) True instantaneous C accumulation rates from northern peatlands as derived from net carbon balance (NCB) in Figure 2a and peatland areas over time. Red circle represents the average of NCB from three peatland sites (c) C isotope composition from atmospheric carbon dioxide (d) Atmospheric CO2 concentrations. Note that high C sequestration in peatlands at 7000–10,000 years ago (Figures 2a and 2b) is likely responsible for the simultaneous CO2 decrease (Figure 2d) and d13CO2 increase (Figure 2c); (Yu et al., Eos, Vol. 92, No. 12, 22 March 2011) The hypothesis that iron (Fe) can act as a limiting micro-nutrient in highnutrient low-chlorophyll (HNLC ) regions (first published 1990) has led to numerous studies that all demonstrate that the addition of Fe to HNLC waters causes an increase in phytoplankton productivity. The broader implication is that in HNLC waters, the addition of soluble Fe(III) can increase the efficiency of the biological pump and promote drawdown of atmospheric carbon dioxide. (H. Planquette et al. / Deep-Sea Research II (2007)) A large diatom bloom peaked in the fourth week after artificial iron fertilization. This was followed by mass mortality of several diatom species that formed rapidly sinking, mucilaginous aggregates of entangled cells and chains. Taken together, multiple lines of evidence lead us to conclude that at least half the bloom biomass sank far below a depth of 1,000 m and that a substantial portion is likely to have reached the sea floor. Thus, iron-fertilized diatom blooms may sequester carbon for timescales of centuries in ocean bottom water and for longer in the sediments. In situ iron enrichment experiments in the open ocean stimulated toxic diatom production (Trick et al., 2010) Domoic acid Recently, several in situ Fe-enrichment experiments e.g. in the Southern Ocean have highlighted the importance of Fe availability for phytoplankton communities in HNLC regions However, such artificial enrichments could lead to adverse side effects such as the occurrence of algal toxins, and reduced oxygen concentration at depth natural iron fertilization off the Crozet Islands in the southern Indian Ocean has been studied whereas natural iron fertilization increased ecosystem biomass, there was no evidence of damage due to reduced oxygen concentration at depth, assuaging the concern that ocean iron fertilization might cause the seafloor to become a biodiversity desert due to lack of oxygen algal toxins were not detectable Bild: www.impactlab.net In the Southern Ocean, sites of natural and continuous fertilization of Fe do exist where there is permanent interaction between water masses and margins of landmasses This phenomenon is called ‘‘the island effect’’. Several studies have inferred Fe-fertilized phytoplankton blooms around island systems in the Southern Ocean Natural sources of Fe, originating either from the islands directly or from the relatively shallow surrounding sediments, relieve Fe stress and therefore promote phytoplankton growth, particularly by the larger cells typically responsible for the export of particulate carbon. Foto: Polar Conservation Organisation H. Planquette et al., Deep-Sea Research II (2007) H. Planquette et al., Deep-Sea Research II (2007) A generally depauperate flora and tundra-like vegetation devoid of trees is typical. Bryophytes are a major component of sub-Antarctic plant communities. The climatic conditions are very suitable for peat formation, and an extensive peat cover has developed on these islands at low altitudes. (van der Putten et al., Palaeogeography, Palaeoclimatology, Palaeoecology 270 , 2008) Natural iron fertilisation around the Crozet Islands southern Indian Ocean Foto: Polar Conservation Organisation Fig. 2. NE–SW cross-section of the Morne Rouge crater (Ile de la Possession, Iles Crozet) with the location of the Morne Rouge sequence and the two lake cores. Radiocarbon dates of the base of the three cores are indicated. (van der Putten et al., Palaeogeography, Palaeoclimatology, Palaeoecology 270 , 2008) 1 Fe nmol L- Ireland: Peatlands cover 13 470 km² or 16.2% of the land surface. Laglera and van den Berg, 2009 Laglera and van den Berg, 2009 Mixing experiments using a 59Fe radiotracer method (filtered Creek water and filtered coastal seawater). The diagram shows the virtual iron concentration in the river water as a function of salinity. Data from Krachler et al., 2005, 2010. asymmetric Flow Field Flow Fractionation - differential refractive index - UV-DAD (=Diodenarray-Detektor) - Fluorescence (3D) - Light Scattering (static & dynamic) - ICP-MS & ICP-OES Burning of peatland (asiacleantech.wordpress.com) Bild: Friends of the Irish Environment Shatura Power Station (Russia) has the largest peat power capacity in the world (Bild: Wikipedia) The Toppila Power Station, a peat-fired facility in Oulu, Finland (Bild: Wikipedia) Autochthonous ligands for Fe(III) in the ocean A.E. Witter et al., 2000 Phytic acid (inositol hexakisphosphate) is the principal storage form of phosphorus in many plant tissues e.g. in edible legumes, cereals, and seed Primary producers in the open ocean are photosynthetic cyanobacteria and eucaryotic microorganisms which are unicellular, freeliving, relying on diffusive fluxes of nutrients Neither group appears to produce siderophores This is an adaption to the dilute pelagic marine environment which promotes large diffusive losses (Hopkinson and Morel, 2009) Heterotrophic marine bacteria synthesize siderophores, however when attached to particles such as marine snow where losses by diffusion can be reduced Inevitably some of the siderophores produced by bacteria are released forming a portion of the natural iron binding ligands in the ocean Rapid production of Fe binding compounds of unknown chemical identity has been detected in response to experimental Fe addition to ironlimited regions of the open ocean (Buck and Bruland, 2007) This response is opposite to that expected of siderophore production, which is upregulated under iron stress and turned off when iron is sufficient One compound secreted by a phytoplanktonic marine eucaryote (Pseudonitzschia), the diatom toxin domoic acid, has been implicated in metal uptake (binds Cu). Domoic acid could play an indirect role in alleviating iron limitation as Cu is involved in diatom iron uptake systems Laglera and van den Berg, 2009: Evidence for geochemical control of iron by humic substances in seawater. A new method, based on the detection of iron-humic substance (Fe-HS) by cathodic stripping voltammetry , was used to determine the iron binding capacity and complex stability of Fe-HS in the seawater. “HS have to be considered as a major ligand for iron, in addition to other ligands that have been proposed for iron” “The covariation of iron and HS in the coastal waters indicates that iron and HS travel together from land to the open sea.” (Irish Sea) Discharge a large volume of freshwater (3300 km3 yr-1) and terrigenous DOM (25 Tg C yr-1) to the shelf and polar surface waters Lignins, which have no autochthonous source in the ocean, have been nevertheless found in low concentrations throughout the entire Arctic, Atlantic, and Pacific oceans Opsahl and Benner, 1997; Benner et al., 2005; Hernes and Benner, 2002; Hernes and Benner, 2006; Louchouarn et al., 2010. Concentrations of total dissolved lignin phenols in polar surface waters: up to 1489 ng/L The large contribution of terrigenous DOM from Arctic rivers is responsible for the elevated concentrations of lignin phenols in polar surface waters Physical transport of terrigenous DOM to the North Atlantic is a major mechanism for its removal from the Arctic This exports compose 25-33% of the terrigenous DOM discharged annually to the Arctic via rivers. The inverse linear relationship between dissolved iron and salinity demonstrates the important role of Arctic rivers in the delivery of dissolved iron to the Arctic Ocean. http://www.copyrightexpired.com/earlyimage/prehistoricl ifebeforekt/n2m_devonianscene.html chemically variable and functionally heterogeneous polymers (colloids) derived from plant decomposition ubiquitous in aquatic ecosystems accounting for up to 95% of dissolved organic carbon (DOC) in freshwater bodies Marine pollution Eutrophication Threat of hypoxia Harmful algal blooms http://www.gulfhypoxia.net/overview/ From: World Ocean Census – A Global Survey of Marine Life 2000 – 2010 Firefly Books, Ontario Through drainage of peatlands in river catchments, dissolved humic substances have been eliminated from river waters Much of the European peat resource has vanished as technology and development have advanced (web site of the Irish Peatland Conservation Council) HS are carbon sources for bacterial degradation HS as food: HS are directly available to filter feeding zooplankton HS shield aquatic organisms from harmful UV radiation HS serve as transport vehicles for bioactive metals HS exert a mild chemical stress upon organisms: An array of oxidative stress symptoms have been reported in several organisms exposed to HS The interaction between metals and naturally occurring humic substances and the thereby induced issues of bioavailability and hydrogeochemical turnover of metal ions in natural waters have been the subject of intense study for decades However metal complexation by humic substances in seawater has become a focus of scientific interest only recently Effect of different iron species on oogonium formation of Laminaria religiosa. Fulvic– iron complex promoted the best oogonium formation rate (79%) of L. religiosa at 35 days compared to amorphous Fe (41%) and control (3%) (n=2). Effect of humic substances on the tetraspores growth of crustose coralline algae (Lithophyllum spp.). FA, fulvic acid ;HA, humic acid. Fe limits phytoplankton growth in extended regions of the ocean Other metals (Zn, Co, Mn) can also occasionally limit phytoplankton growth in the ocean but may play a more important role in regulating the composition of phytoplankton because of large differences in trace metal requirements among species Likewise, low Fe availability can have a critical influence on the composition and structure of algal communities, because of differences in requirements among species It serves essential metabolic functions in: photosynthetic electron transport respiratory electron transport nitrate and nitrite reduction sulfate reduction N2 fixation detoxification of reactive oxygen species Fe requirements are influenced by a number of factors such as light intensity, nitrogen source (NH4+, N2, NO3-, urea) , CO2 availability etc. Fe limits phytoplankton growth in high-nitrate low-chlorophyll waters (25% of the word‘s ocean) Fe limits nitrogen fixation by diazotrophs in nitrate-poor low-latitude waters Together, low Fe concentrations limit productivity in about 50% of the world ocean Iron deficiency limits the drawdown of CO2 from the atmosphere by phytoplankton Fe is therefore a critical component of the Earth‘s climate system atmospheric dust deposition from arid regions volcanic ash deposition, extraterrestrial dust ice melting hydrothermal vents (not a significant iron source for microbes in the euphotic zone) upwelling offshore eddy transport of iron-rich coastal waters The majority of dissolved Fe in river water exists as small colloid particles (Fox,1988; Dai and Martin, 1995; Wen et al., 1999). Flocculation of these colloids, due to the change in ionic strength upon mixing of river water with seawater, causes a massive removal of the Fe (Sholkovitz, 1978; Sholkovitz et al., 1978). rrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrr r short residence time of iron, external sources and sinks We propose that with peat-bog derived HS as an important carrier mechanism for riverine Fe, the influence of this Fe source reaches further out to sea than previously expected Oligomeric lignin phenols as important land-derived iron chelators in the ocean? The concentrations of lignin phenols in the open ocean are small, but may be sufficient to keep Fe soluble Cinnamic Acid Vanillin Syringealdehyde Sinapic Acid Ferulic Acid Acetosyringone P-Coumaric Acid Syringic Acid (Opsahl and Benner, 2000) Neben Cellulose die häufigsten organischen Verbindungen auf unserem Planeten. Etwa 20 % bis 30 % der Trockenmasse verholzter Pflanzen besteht aus Ligninen. Die Gesamtproduktion der Lignine wird auf 20 Milliarden Tonnen pro Jahr geschätzt. Lignine sind wesentlich für die Druck-Festigkeit von pflanzlichen Geweben. Ihre „Erfindung“ ermöglichte die Evolution der landlebenden Pflanzen. Werden nur langsam und nur aerob von bestimmtem Pilzen und Bakterien abgebaut. Quelle: http://commons.wikimedia.org/wiki/File:Monolignols_ general.svg Cumarylalkohol (1), Coniferylalkohol (2) Sinapylalkohol (3) Inorganic iron (the sum of free hydrated and hydrolyzed ferric iron species) is directly available for phytoplankton uptake, whereas humic-iron complexes have been thought in the past to be not bioavailable (Hudson and Morel, 1993) The Amur River plume which is transported by the east Sakhalin current is a major source of bioavailable iron to the Okhotsk Sea (Yoshimura et al., 2010) Amur River http://travel.webshots.com/photo/1385175464065970692DLkGnB This study concluded that hydrophobic DOM, as chelating agent, is a biologically important component in natural waters Jiho Lee et al., Ecotoxicology and Environmental Safety 72 (2009) 335–343 Riverine Fe-HS enhance ocean productivity and remove carbon dioxide from the atmosphere thereby mitigating climate change and ocean acidification









