Bioluminescence
advertisement
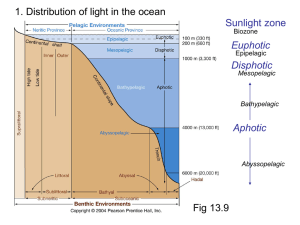
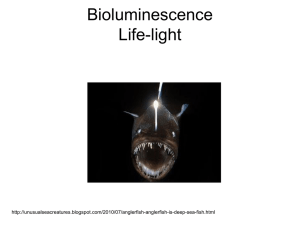
Bioluminescence By: KN What is Bioluminescence? Bioluminescence is the production and emission of light by any living organism Glowing animals, typically create light through luminescence Chemical compounds are mix together to produce a glow Most of the energy generated is emitted as light rather than as heat and thus known as cold light Bioluminescent Life Forms Bioluminescence has been found across a broad range of the major groups of organisms from bacteria and protists to squid and fishes, with numerous phyla in between Terrestrial species (e.g. fireflies) have the same ability Bioluminescence is the only source of light for the underwater creatures About 90% of the organisms that live in the ocean have the capability to produce light These creatures produce light for a variety of reasons: Finding or attracting prey Defence against predators Communication Mate attraction/recognition Most abundant bioluminescent dinoflagellate, Noctiluca scintillans Below: Flashes when disturbed Anglerfish The female anglerfish attracts its prey by dangling a lighted “lure” that extends from the top of its head to the front of its gaping mouth This lighted lure contains bioluminescent bacteria Male anglerfishes are attracted to the females by their odor and distinctive light displays Luciferin & Luciferase Photoprotein Raphael Dubois, professor of physiology at the University of Lyons, France, and director of the Marine Laboratory at Tamaris-sur-Mer Major Luminescence Systems 1. Bacterial Luciferin is a reduced riboflavin phosphate and found in bacteria, some fish, and squid. 2. Dinoflagellate Luciferin is thought to be derived from chlorophyll because it has similar structure and is found in dinoflagellates and euphasiid shrimp. 3. Vargulin (Cypridina) is found in the ostracod Vargula. Was one of the first marine luciferins chemically well understood. Ostracods synthesize their luciferin from tryptophan, isoleucine, and arginine 4. Coelenterazine (Aequorin) is the most common luciferin; it is found in many phyla- the radiolarians, ctenophores, cnidarians, squids, copepods, chaetognaths, and some fish and shrimp and very likely, the hydrozoa (jellyfish, Aequorea). 5. Firefly luciferin which requires adenosine triphosphate (ATP) - the energy currency of cells- as a cofactor in its reactions. Aequorea victoria Coelenterazine has been found to be the light-emitter in an ever-growing list of bioluminescent species representing nine phyla The components of this molecule was first discovered by a young Japanese Fulbright Scholar and biochemist named Osamu Shimomura In 1960, Shimomura was received an invitation to Princeton to further study the bioluminescent jellyfish Aequorea victoria, specifically in isolating the luminous reactants and purifying the bioluminescent properties of the jellyfish Shimomura purified a photoprotein he called Aequorin that when activated by calcium emitted bright blue light (469 nm). This form of calcium-activated bioluminescence reaction does in fact involve a luciferin, called coelenterazine that binds to the Aequorin rapidly and tightly but produces light only when calcium is present. Aequorin GFP (Green Fluorescent Protein) Applications of Bioluminescence The protein, GFP can be found in the photo organs of Aequorea victoria. In this certain process, the blue light is absorbed by the GFP, and converted into visible green light This exchange of energy is a process called bioluminescence resonance energy transfer. Green fluorescent protein is expressed in almost any organism: plants, frogs, fishes, mice, yeast and more. Now researchers have developed ways to watch processes that were previously invisible, such as the development of nerve cells in the brain or how cancer cells spread. Above: A live mouse containing the gene for red fluorescent protein in the brain Left: A live mouse with lung tumors labeled with red and green fluorescent protein Bioluminescent Imaging (BLI) Bioluminescence imaging has been emerging as a tool commonly used for preclinical cellular and molecular imaging in small animals. This type of imaging is performed in vivo and involves an injection of luciferin into an animal using a type of luciferase enzyme, most frequently the firefly luciferase which is widely used as a marker for gene expression in living organisms (A study done in vivo looks at how the body responds to a particular substance) Molecular imaging requires a type of luciferase, the most frequently used: Firefly (Photinus pyralis) luciferase. In addition to the enzyme-substrate reaction, it requires cofactors such as oxygen, magnesium and ATP that produces an electronically excited oxyluciferin Firefly luciferase remains the preferred enzyme for bioluminescence molecular imaging because its reaction with luciferin produces more light (emits light with a broad emission spectrum and a peak at 560 nm) Yet many factors can influence bioluminescence measurements which makes this a highly sensitive method for small-animal molecular imaging (a) One mice injected with a type of virus and monitored using the (b) PET scan and the (c) optical imaging (BLI) where you can see the exact location of the virus in the body Conclusion Although bioluminescence is common, there are still many unknown aspects of this phenomenon to be discovered. Animals have lots of methods for producing and using light, in ways like attracting a mate, attracting prey, deterring predators, and in hunting and with that humans are beginning to understand the significance of bioluminescence Scientists have only begun to further discover and implement useful tools that can be used in curing and tracing the path of disease, in analyzing cellular dynamics, and to continue to improve the inexplicable quality of human life. References Haddock, S., & Case, J. F. (1997). In The Bioluminescence Webpage. Retrieved February 17, 2012, from http://www.lifesci.ucsb.edu/~biolum/ Knight, J. (1998). Deep Sea Bioluminescence. In Creatures of the Deep Sea. Retrieved February 17, 2012, from http://www.seasky.org/deep-sea/biolumiscence.html Macody, . (1997). How Does Bioluminescence Work? . In The Physiology of Bioluminescence . Retrieved February 20, 2012, from http://www.bio.davidson.edu/Courses/anphys/1999/Cody/howworks.html Latz, M.I., J.C. Nauen, and J. Rohr. Bioluminescence Questions and Answers (2004). Retrieved February 20, 2012, from http://siobiolum.ucsd.edu/Biolum_q&a.html November 2 SciCafe: Q&A with Bioluminescence and Biofluorescence Experts. (2010, October 27). In American Museum of Natural History. Retrieved March 12, 2012, from http://www.amnh.org/news/2011/10/november-2scicafe-qa-with-bioluminescence-experts/#more-5266 Lynch, T. (1999). The luciferase-luciferin reaction in Photinus pyralis. In Bioluminescence in Fireflies. Retrieved March 12, 2012, from http://www.byteland.org/naturalist/dubois.htm Steven H.D. Haddock, Mark A. Moline, and James F. Case. Bioluminescence in the Sea (Vol. 2, pp. 443-493). (2010). N.p.: Annual Reviews. Retrieved March 12, 2012, from http://www.annualreviews.org/doi/full/10.1146/annurev-marine-120308-081028 Shimomura, O., & Johnson, F. H. (1975, April). Chemical nature of bioluminescence systems in coelenterates. In Proceedings of the National Academy of Sciences of the United States of America . Retrieved March 12, 2012, from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC432574/ Luker, G. D., & Luker, K. E. (2007, December 12). Optical imaging: current applications and future directions. Retrieved March 12, 2012, from http://www.ncbi.nlm.nih.gov/pubmed/18077528 Luker, G. D., & Luker, K. E. (2008, January). Optical Imaging: Current Applications and Future Directions. In Focus on Molecular Imaging. Retrieved March 12, 2012, from http://jnm.snmjournals.org/content/49/1/1.full.pdf Klug, T. (2001, June 8). In Uses of Bioluminescence (Final). Retrieved April 23, 2012, from http://jrscience.wcp.muohio.edu/fieldcourses01/MarineEcologyArticles/UsesofBioluminescenceFina.html Zimmer, M. (2008). History. In GFP- Green Fluorescent Protein. Retrieved April 23, 2012, from http://www.conncoll.edu/ccacad/zimmer/GFP-ww/shimomura.html Hole, W. (2008, October 8). MBL Scientist Osamu Shimomura Wins Nobel Prize in Chemistry for Discovery Of Green Fluorescent Protein. In Marine Biological Laboratory. Retrieved April 23, 2012, from http://www.mbl.edu/news/features/shimomura.html Pieribone, V., Gruber, D. F., Cotton, A., & Poole, F. (2005). A Glow in the Dark: The Revolutionary Science of Biofluorescence (pp. 14-154). Cambridge, Massachusetts, MA: The Belknap Press of Harvard University Press. Rich, P. B., & Douillet, C. (Eds.). (2009). Bioluminescence: Methods and Protocols (2nd ed., Vol. 1, pp. 7-23). Chapel Hill, NC: University of Carolina at Chapel Hill. Pictures http://ocean.si.edu/slideshow/bioluminescent-animals-photo-gallery http://www.flickr.com/photos/36494393@N00/397899023/ http://www.bioart.co.uk/lux/intro.html http://www.whoi.edu/page.do?pid=80696&i=8865 http://joy-of-bioprocess.blogspot.ca/2007_11_01_archive.html http://www.lonza.com/products-services/bio-research/assay-solutions/cell-basedassays/clonetics-primary-sensors.aspx www.nature.com http://www.sciencedirect.com/science/article/pii/S0006291X09019925 http://www.annualreviews.org/action/showPopup?citid=citart1&id=f3&doi=10.1146%2Fa nnurev-marine-120308-081028 http://science.howstuffworks.com/environmental/life/zoology/all-aboutanimals/bioluminescence.htm http://www.annualreviews.org/action/showPopup?citid=citart1&id=f4&doi=10.1146%2Fa nnurev-marine-120308-081028 http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2008/shimomuraautobio.html http://foreignerinformosa.typepad.com/the_foreigner_in_formosa/science_technology/ http://www.norbertwu.com/nwp/behaviors-themes/animals_feeding_web/gallery-05.html