step molecular
advertisement

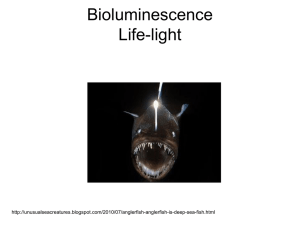
The Wonders of Bioluminescence During the day, the sun lights the streets and alleys, meadows and grasslands, the surface of dark blue seas. At night, moons, stars, and streetlamps illuminate the darkness. In some cases, animals such as fireflies, glowworms, click beetles, jellyfish, and squid can even produce their own light through chemical reactions. Light production in fireflies is due to a type of chemical reaction called bioluminescence, a process by which an organism produces and emits light through conversion of chemical energy to light energy. The components involved in the production of light in a firefly include luciferin, molecular oxygen, and adenosine triphosphate (ATP), which serves as the general source of energy. Luciferase or luminase are generic terms for enzymes that catalyze the process of bioluminescence in nature and are also involved in the light production of a firefly. Luciferase catalyzes a variety of reactions using a variety of different substrates. In fireflies, luciferase catalyzes an oxidative reaction involving ATP, luciferin and molecular oxygen. The result of the reaction yields an electronically excited oxyluciferin species. One proposed mechanism of the luciferin-luciferase reaction involves activation of the carboxylate group of luciferin by ATP to form the adenylated luciferin. Then the alpha subunit is lost, allowing the reaction with molecular oxygen. Cleavage of the dioxetanone ring yields the excited state of oxyluciferin. The catalytic mechanism of firefly luciferase. Firefly luciferase acts not only as a mono-oxygenase but also a synthetase to generate the acyl adenylate. The catalytic mechanism of firefly luciferase is a multi-step process: Step 1: The firefly D-luciferin is transformed to the enzyme-bound luciferyl adenylate, releasing the inorganic pyrophosphate when ATP and magnesium are present. The adenosine monophosphate, AMP, is the substrate for the oxidation of firefly luciferase. Step 2: Although firefly luciferase functions as the mono-oxygenase, it behaves in a very unusual manner without the apparent involvement of a metal or cofactor. The oxygen generates a ringed species which later transforms into the electronic excited state and yields carbon dioxide. The excited state of Luc⋅ Oxyluciferin and the carbon dioxide each contains an oxygen atom from molecular oxygen. Step 3: The rapid loss of energy of the excited Luc⋅ Oxyluciferin to its ground state results in emission of light. Because every reacted luciferin emits a photon, a majority of the energy is used towards light production rather than heat production. This bioluminescence, also known as "cold light," is 96% efficient, far more efficient than an incandescent light with an efficiency rating of 10%. At the same time, fireflies can also use dehydroluciferin to yield the acyl adenylate and inorganic pyrophosphate. This step which has no further steps is considered as the limitation step for step 1 to inhibit the activity of the enzyme. All known bioluminescence reactions require oxygen and the intermediacy of peroxides; however, luciferases constitute a diverse group of unrelated enzymes acting upon chemically different luciferins and employing a variety of cofactors. The glow of fireflies serves many purposes. It acts to beget life, and it acts to protect it. For example, many adult fireflies have flash patterns unique to their species and use them to identify other members of their species as well as to distinguish between members of the opposite sex. Several studies have shown that female fireflies choose mates depending upon specific male flash pattern characteristics. Higher male flash rates, as well as increased flash intensity, have been shown to be more attractive to females in two different firefly species. Furthermore, the larvae use their glows as warning signals to communicate their distastefulness. The ability of fireflies to control the rate at which they emit light are explained by two theories: the Oxygen Control Theory and the Neural Activation Theory. As mentioned previously, oxygen is required in the production of light. On one hand, the Oxygen Control Theory explains how fireflies are able to regulate oxygen intake, therefore controlling the rate at which light is emitted. The Neural Activation Theory, on the other hand, explains how fireflies have neural-controlled structures structures called tracheal end cells that facilitate the chemical reaction. Insight into the mechanisms that govern luciferase allows scientists to apply bioluminescence in the other areas of study. For example, Christopher Contag, professor of microbiology and immunology at Stanford University, proposed using bioluminescence as an indicator of life. Since ATP is a primary component of living organisms and a requirement in the function of luciferase, bioluminescent activity can indicate whether or not ATP is present in a culture or specimen. Additionally, NASA also considered using this technique to determine the possibility of past lives in other planets by testing for ATP in planet samples. Not only is bioluminescence a good indicator of life, it also serves as a good marker. To test the strength of an antibiotic, luciferase can be added to tuberculosis samples along with the antibiotic. If the antibiotic is potent enough, the bacteria would all die. If not, some bacteria would still be alive and divide as indicated by a glow when luciferase reacts with oxygen and ATP to produce light. Green fluorescent protein (GFP) from jellyfish are also great markers for determining the location of proteins. When hit with blue light, GFP glows green. Therefore, proteins of interest may be detected by staining cells with fluorescently labeled antibodies. Bioluminescence is a natural wonder with the potential to be incorporated into applications that can help further scientific research. However, it is sometimes overlooked. By exploring the production and the different purposes of bioluminescence among organisms like the firefly, one can appreciate the natural beauty that our earth has to offer us.