Document
advertisement

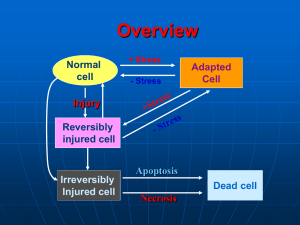
Alteration Degenerations Nekrosis Lecture on pathological anatomy for the 3-rd year students T.Filonenko Alteration. Cell injury. Alteration is the pathological changes of cellular structure, extracellular matrix, tissue and organs which are accompanied by violation of their vital functions. The cellular morphologic changes induced by various stimuli can be divided into: 1. Patterns of acute cell injury — reversible and irreversible cell injury leading to necrosis or apoptosis 2. Subcellular alterations that occur largely as a response to more chronic or persistent injurious stimuli 3. Intracellular accumulations of a number of substances — lipids, carbohydrates, proteins—as a result of derangements in cell metabolism or excessive storage. Reasons of development of alteration 1. hypoxia 2. chemical agents and drugs 3. physical agents 4. microbiological agents (bacteria, viruses, 5. 6. 7. 8. fungies) immune mechanisms genetic defects (apoptosis) nutritional imbalances aging Classification of degenerations 1. Сlassification in depending on localization of metabolism: parenchymal stromally - vascular mixed 2. Classification in depending on deposition of protein, lipids, carbohydrate , mineral (on predominance of the broken exchange): Proteinous (Dysproteinoses) Fatty (lipidoses) Carbohydrate Mineral Pigmental 3. Classification in depending on prevalence of process: Local System 4. Classification in depending on an origin: Acquired Hereditary Categories of intracellular accumulations 1. a normal cellular constituent accumulated in excess, such as water, lipid, protein, and carbohydrates; 2. an abnormal substance, either exogenous, such as a mineral, or a product of abnormal metabolism; 3. a pigment or an infectious product. Parenchymal dysproteinoses, mechanisms. Cell Denaturation and coagulation of cytoplasmic proteins Hydratation and colliquation of cell’s cytoplasm Hyaline-drop dystrophy Hydropic dystrophy Focal coagulative necrosis of cell Focal colliquative necrosis of cell (balloon dystrophy) Total colliquative necrosis of cell Total coagulativeNecrosis of cell The most frequent localization of intracellular accumulations of proteins, lipids and carbohydrates is myocardium (cardiomyocytes), liver (hepatocytes), kidneys (nephrocytes). Types of intracellular parenhymatous degenerations Granular Hyaline-drop Hydropic (vacuolar, balloon) Keratoid (horney) Here are Mallory bodies Parenhymatous fatty degenerations This liver is enlarged and has a pale yellow appearance. It is greasy to touch. It is called “Goose liver”. Microscopically: there are numerous lipid vacuoles in the cytoplasm of hepatocytes. Extracellular proteinous degenerations 1. Mukoid swelling 2. Fibrinoid changes 3. Hyalynosis 4. Amyloydosis Mucoid swelling It is disorganization and swelling of perivascular extracellular matrix (disorganization of connective tissue) due to increased vascular permeability, plasmorrhagia and deposition of glucosaminoglycans (GAG). Microscopically: there is the phenomenon of metachromasia. That is basophylic color of basic substances. Collagen fibers save the structure, but swell and undergo to fibrillar destructure. Gross appearance: tissue or organ is saved. Process is convertible. Fibrinoid changes It is deep and irreversible disorganization of connective tissue, in basis of which destruction of basic substances and fibers. It is accompanied by the sharp increase of permeability of vessels and formation of fibrinoid masses. Microscopically: the bands of collagen fibers are homogenous, impregnated with plasma proteins. Outcomes: fibrinoid necrosis, hyalinosis, sclerosis. Hyaline change It is an alteration within cells or in the extracellular space, which gives a homogenous, glassy, pink appearance in routine histologic sections stained with H&E. Hyalinosis is classified according to its localization: Vascular hyalinosis (arteries are thickened with sharply narrowed or obliterated lumen) Hyalinosis of connective tissue is usually localized; it develops in scars, adhesions, in the areas of chronic inflammation (e.g. “glazed spleen”). The outcome of hyalinosis is irreversible. Functional significance of hyalinosis is different: • Vascular hyalinosis may lead to atrophy or sclerosis, infarction of organs. • Local hyalinosis in the cardiac valves results in heart defects. Amyloidosis Amyloidosis is the term used for a group of diseases characterized by extracellular deposition of febrillar proteinaceous substance called amyloid. Nature and etiology Amyloid is composed of 2 main types of complex proteins: Fibril proteins comprising about 90% of amyloid. P-component comprising about 10% of amyloid. Of the 15 biochemically distinct forms of amyloid proteins that have been identified, two are the most common: One, called AL (amyloid light chain) is derived from plasma cells (immunocytes) and contains immunoglobulin light chains. The other, designated AA (amyloid-associated), is a unique nonimmunoglobulin protein synthesized by the liver. Classification of amyloidosis A. Systemic Amyloidosis 1. Primary amyloidosis (idiopathic) is the defect of primary mesodermal tissue 2. Secondary amyloidosis (acquired, reactive) is complication of chronic diseases (chronic infections, malignant tumors) 3. Familial amyloidosis (inherited, genetic) is predisposition of certain ethnic groups (periodic illness). B. Localized Amyloidosis 1. Senile amyloidosis 2. Endocrine amyloidosis Diagnosis of amyloidosis Histologic examination of biopsy material is the commonest and confirmatory method for diagnosis in a suspected case of amyloidosis. If renal manifestations are present, kidney is the preferred site for biopsy. Other sites such as rectum, gingiva, and more recently abdominal fat, are biopsied and are followed by Congo red staining for confirmation. Pathology Systemic amyloidosis (AA) related to chronic inflammation tends to involve parenchymatous organs, such as kidneys, spleen, liver, and adrenals. While amyloidosis (AL) related to myeloma tends to affect mesodermal or other tissues, such as heart, gastrointestinal tract, peripheral nerves, skin, and tongue. Grossly: Organs extensively infiltrated by amyloid are usually enlarged and have a pale, waxy ("lardaceous") or varnished appearance and firm consistency. The iodine test for amyloid is done by applying iodine solution to the washed cut surface of the organ: amyloid typically stains mahogany-brown, and this color reaction changes to blue ( a "starch-like" reaction) after the application of dilute sulfuric acid Primary amyloidosis of kidneys. Grossly, amyloid kidneys are usually enlarged, pale, and smooth surfaced and have a firm consistency. On cortical transaction, the glomeruli (visible as pink dots in the normal kidney) may be seen as enlarged, waxy, gray dots. Microscopically: the amorphous pink deposition of amyloid may be found in and around arteries, in interstitium, or in glomeruli. A Congo red stain will demonstrate the red material to be amyloid. H&E Congo red Amyloidosis of myocardium This section of myocardium demonstrates amorphous deposits of pale pink (H&E ) or red (Congo red) material between myocardial fibers. Amyloidosis is a cause for "infiltrative" or "restrictive" cardiomyopathy. Amyloidosis of the spleen Amyloidosis of the spleen has two different anatomical patterns. Most commonly, the amyloid deposits is limited to the splenic follicles, resulting in the gross appearance of a moderately enlarged spleen dotted with gray nodules (so called "sago" spleen). Alternatively, the amyloid deposits may spare the follicles and mainly infiltrate the red pulp sinuses, producing a large, firm spleen mottled with waxy discolorations ("lardaceous" spleen). Amyloidosis of adrenal gland Amyloid deposits surround, compress, and replace some cortical cells and infiltrate the wall of a small blood vessel. Congo red. Amyloidosis of the tongue Amyloid infiltrates the capillary walls and narrows the lumens of some of them. H&E. Amyloidosis of the liver The hepatic parenchyma is infiltrated and replaced by nodular accumulations of amyloid (pink). H&E. Clinical manifestations of amyloidosis Stromal vascular fatty degenerations • Stromal fatty infiltration is the deposition of mature adipose cells in the stromal connective tissue. • The condition occurs most often in patients with obesity. Classifications of Obesity 1. According to the etiology : Primary (idiopathic) and Secondary. 2. There are several types of secondary obesity: Alimentary, Cerebral, Endocrine, Hereditary in Gierke’s disease. 3. According to the patient's appearance: symmetrical, upper, medial, and lower. 4. According to morphological peculiarities of adipose tissue: • In hypertrophic type adipose tissue enlarges due to increased volume of fatty cells • In hyperplastic due to increase in their number. PATHOLOGY OF PIGMENTS Pigments are colored substances, some of which are normal constituents of cells where as others are abnormal and collect in cells only under special circumstances. Pigments are generally classified into two broad categories: 1. Endogenous pigments, which are normal constituents of cells and tissues; 2. Exogenous pigments introduced into the body from environment. 1. 2. 3. Classification of endogenous pigments Hemoglobinogenic pigments: Physiologic pigments: Ferritin, Hemosiderin, Bilirubin Pathologic pigments: Hematoidin, Hematin, Porfirin Proteinogenic (melanin). Lipidogenic (lipofuscin). Hemosiderosis Local hemosiderosis is characterized by local breakdown of red cells in tissues, e.g. in internal hemorrhage. Mechanism of local hemosiderosis is extravascular hemolysis. It occurs regularly around areas of bruising and hemorrhage. In the lungs hemosiderin-laden macrophages (siderophages) are appropriately referred to as “heart failure cells”. Visceral siderosis (systemic hemosiderosis). A Prussian blue reaction is seen in this iron stain of the liver to demonstrate large amounts of hemosiderin that are present in hepatocytes and Kupffer cells. Mechanism of systemic hemosiderosis is intravascular hemolysis. It is seen in the liver, spleen and sometimes in kidneys in cases of hemolytic anemia, in patients requiring repeated blood transfusion, in patients with chronic ineffective erythropoiesis. The pigment imparts a deep brown color to tissues and organs when it is present in high concentrations. Pathology of bilirubin’s metabolism. Jaundice. Types of jaundice: 1. Prehepatic jaundice (Hemolytic jaundice) is characterized by lysis of the red blood cells in a variety of conditions. 2. Intrahepatic jaundice (Hepatocellular jaundice) - results from failure both of hepatocytes to conjugate bilirubin and of bilirubin to pass through the liver into the intestine. Both of conjugated bilirubin and unconjugated bilirubin increase its amount in blood. The liver is light yellowish-green color of saffron (“saffron liver”). 3. Posthepatic jaundice (Obstructive jaundice) - results from an obstruction of the passage of conjugated bilirubin from hepatocytes to the intestine. Conjugated bilirubin is water-soluble and is excreted in the urine. The liver is dark green. In the liver, bile pigments may appear: • As bile pigment droplets in the hepatocytes. • As bile impregnations in necrotic areas. • As bile casts (bile capillaries, cholangioles, or bile canaliculi). • In Kupffer’s cells. The yellow-green globular material seen in small bile ductules in the liver here is bilirubin pigment. Calcium metabolism disturbances This is dystrophic calcification in the wall of the stomach. At the far left is an artery with calcification in its wall. There are also irregular bluish-purple deposits of calcium in the submucosa. Calcium is more likely to be deposited in tissues that are damaged. Here is so-called "metastatic calcification" in the lung of a patient with a very high serum calcium level (hypercalcemia). Cells Death It is the premature death and destruction of cell in the living organism under action of factors of critical damage Classification of Cells Death, based on the mechanism of development: necrosis pathogenic inducted apoptosis immunological elimination of cells. NECROSIS If the acute or chronic injury to which a cell must react is too great, the resulting changes in structure and function lead to the death of cells. Death of the cells and tissues in a living organism is called Necrosis. According to the cause of necrosis there are the following types of necrosis: traumatic necrosis; toxic necrosis; trophoneurotic necrosis; allergic necrosis; vascular or ischemic necrosis. Main types of necrosis Two essential changes bring about irreversible cell injury in necrosis - cell digestion by denaturation of proteins and lytic enzymes. coagulative necrosis develops (during denaturation of proteins ). liquefactive necrosis is a progressive catalysis of cell structures (during enzymic digestion). Liquefactive necrosis is typical of organs in which the tissues have a lot of lipid (such as brain) or when there is an abscess with lots of acute inflammatory cells whose release of proteolytic Both of these processes require hours to develop Clinic-morphological forms of necrosis of organs: 1) Gangrene – total necrosis of the organ, reported with the external environment: dry – at the thrombosis of arteries, an organ acquires the black coloring moist (wet) – at the thrombosis of arteries and veins + influencing of putrid bacteria. gas gangrene bedsore is a type of gangrene, death of the tissue under the influence of pressure (sacral area, buttocks, great trochanter). It is trophoneurotic necrosis of the bed- patients noma – widespread necrosis of soft tissue of person. 2) Sequester – fragment of dead tissue, which can’t be autolysed, replaced by connective tissue and which is localized among alive tissue 3) Infarction – vascular or ischemic necrosis; 4) Fat necrosis 5) Caseous necrosis 6) Fibrinoid necrosis. Infarction This is an example of coagulative necrosis. This is the typical pattern with ischemia and infarction (loss of blood supply and resultant tissue anoxia). Here, there is a wedgeshaped pale area of coagulative necrosis (infarction) in the renal cortex of the kidney. The contrast between normal adrenal cortex and the small pale infarct is good. The area just under the capsule is spared because of blood supply from capsular arterial branches. This picture illustrates the shape and appearance of an ischemic (pale) infarct well. Liquefactive necrosis At high magnification, liquefactive necrosis of the brain demonstrates many macrophages at the right which are cleaning up the necrotic cellular debris. Grossly, the cerebral infarction at the upper left here demonstrates liquefactive necrosis. Eventually, the removal of the dead tissue leaves behind a cavity Gangrene This is gangrene. In this case, the toes were involved in a frostbite injury. This is an example of "dry" gangrene in which there is mainly coagulative necrosis from the anoxic injury. This is gangrene of the lower extremity. In this case the term "wet" gangrene is more applicable because of the liquefactive component from superimposed infection in addition to the coagulative necrosis from loss of blood supply. This patient had diabetes mellitus. Fat necrosis This is fat necrosis of the pancreas. Cellular injury to the pancreatic acini leads to release of powerful enzymes which damage fat by the production of soaps, and these appear grossly as the soft, chalky white areas seen here on the cut surfaces. Microscopically, fat necrosis is seen here. Though the cellular outlines vaguely remain, the fat cells have lost their peripheral nuclei and their cytoplasm has become a pink amorphous mass of necrotic material. Caseous necrosis This is more extensive caseous necrosis, with confluent cheesy tan granulomas in the upper portion of this lung in a patient with tuberculosis. The tissue destruction is so extensive that there are areas of cavitation (cystic spaces) being formed as the necrotic (mainly liquefied) debris drains out via the bronchi. Microscopically, caseous necrosis is characterized by acellular pink areas of necrosis, as seen here at the upper right, surrounded by a granulomatous inflammatory process. Fibrinoid necrosis Sometimes the small arteries and arterioles can be damaged so severely in malignant hypertension that they demonstrate necrosis with a pink fibrin-like quality that gives this process its name - fibrinoid necrosis. Phase of necrosis Many nuclei have become pyknotic (shrunken and dark). It process is called karyopicnosis. After that karyorrhexis (fragmentation) develops. Nucleus is decomposed into small granules. Also may be develops karyolysis, when the nucleus dissolves. The cytoplasm and cell borders are not recognizable. The outcomes of necrosis Regeneration of tissues – replacement of the dead tissue with a new one; Incapsulation – formation of the connective tissue capsula around necrotic area; Organization – replacement of the dead tissue with connective tissue; Petrification – replacement of the dead tissue with calcium salts; Incrustation – replacement of the dead tissue with any other salts exept calcium; Ossification – the formation of the bone tissue in the necrotic area; Hyaline change – the appearance of the hyaline-like substance in the necrotic area; Sequestration – formation of sequester; Mutilation – spontaneous tearing away of the dead tissue; Cystic formation. Suppuration fusion of necrotic tissues