glucose dysregulation - Josephine Carlos
advertisement
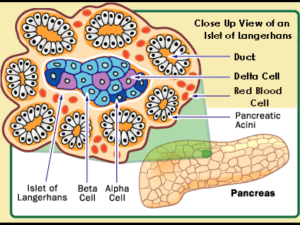
Current Diagnosis and Mechanisms of Glucose Dysregulation Dr. Josephine Carlos-Raboca Chief, Section of Endocrinology, Diabetes & Metabolism Makati Medical Center Immediate Past President, PSEM Outline Overview of glucose regulation Stages of Dysglycemia (glucose dysregulation) Current Diagnostic Criteria for dysglycemia Mechanisms of Glucose Dysregulation Summary Glucose Metabolism Tightly regulated to maintain adequate plasma levels Major hormones insulin glucagon incretins Major organs islet cells of pancreas insulin sensitive organs: liver, muscle, fat intestines Kidneys Modulator Endocannabinoid System Islet as an Organ: Role of Pancreatic Islets in Normal Glucose Homeostasis Islet of Langerhans ~ 3,000 cells 200 µm 75% Beta cells 25% non-Beta cells Micrograph: Lelio Orci, Geneva Beta and Alpha Cells in the Pancreas of Normal Individuals Beta Cells • • • Alpha Cells Comprise about 50% of the endocrine mass of the pancreas1 Produce insulin and amylin2 • Insulin released in response to elevated blood glucose levels2 • • Comprise about 35% of the endocrine mass of the pancreas1 Produce glucagon2 Glucagon released in response to low blood glucose levels2 leading to increase in glucose 1. Cabrera O et al. PNAS. 2006;103:2334–2339. 2. Cleaver O et al. In: Joslin’s Diabetes Mellitus. Lippincott Williams & Wilkins; 2005:21–39. Insulin Production Primary regulators for insulin biosynthesis glucose glucagon incretins- GLP-1, GIP Inhibits insulin biosynthesis catecholamine somatostatin Glucagon Main regulator- glucose amino acids incretins Insulin fatty acids ketones Insulin and Glucagon Regulate Normal Glucose Homeostasis Glucagon (Alpha cell) (–) (–) Pancreas (+) Insulin (Beta cell) (–) (+) (+) Glucose uptake Glucose output Blood glucose Liver Muscle and adipose tissue Porte D Jr et al. Clin Invest Med. 1995;18:247–254. Adapted from Kahn CR, Saltiel AR. Joslin’s Diabetes Mellitus. 14th ed. Lippincott Williams & Wilkins; 2005:145–168. GUT and GUT Hormones Na ATP channels – absorption of glucose Incretins Incretins Regulate Glucose Homeostasis Through Effects on Islet-Cell Function Ingestion of food Insulin in glucose-dependent way from β cells (GLP-1 and GIP) GI tract Release of incretin gut hormones Pancreas INSULIN β cells α cells Active GLP-1 and GIP Blood Blood glucose control control ↓ GLUCAGON Glucagon Inactive GLP-1 (9-36) and GIP (3-42) Increased peripheral glucose uptake in glucosedependent way from α cells (GLP-1) Decreased hepatic glucose output Adapted from Brubaker PL, Drucker DJ. Endocrinology. 2004;145:2653-2659;Zander M et al. Lancet. 2002;359:824-830; Ahrén B. Curr Diab Rep. 2003;3:365-372; Buse JB et al. In Larsen PR et al, eds.: Williams Textbook of Endocrinology. 10th ed. Philadelphia, PA: Saunders; 2003:1427-1483. The endocannabinoid system is a modulatory system Endocannabinoids: Synthesized on demand from lipid precursors in postsynaptic cell CB1 receptors: Play a key role in energy balance and lipid and glucose metabolism Di Marzo V et al, 2005; Di Marzo V et al, 1998; Wilson R et al, 2002 Central and peripheral targets of the endocannabinoid system Brain Peripheral tissues Adipose tissue Hypothalamus: ^ hunger Nucleus accumbens: ^ motivation to eat Increased food intake Increased fat storage Liver GI tract Muscle ^ Insulin resistance HDL-cholesterol ^ Triglycerides Glucose uptake Adiponectin HDL: high-density lipoprotein Bensaid M et al, 2003; Pagotto U et al, 2005; Osei-Hyiaman D et al, 2005; Di Marzo V et al, 2005; Liu YL et al, 2005 Regulation of glucose Homeostasis Na dependent transporters in proximal tubules of kidneys cotransport glucose with sodium maintained by Na+/K+-ATPase ion pump Glucose homeostasis Is a balance of glucose appearance and disappearance Glucose appearance: endogenous glucose production (liver, muscle and kidneys) exogenous sources (GIT) affected by feeding signals Glucose disappearance peripheral uptake from liver, muscle and fat Current Diagnosis of Prediabetes and Diabetes Definition of Diabetes A metabolic dysregulation Hallmark: hyperglycemia Basic defects: Islet cell dysfunction Insulin insensitivity Impaired action of insulin on target tissues Definition of Diabetes Chronic hyperglycaemia associated with longterm damage to: Eyes Kidneys Nerves Heart and blood vessels Hyperglycemia Stages Type Type 1 * Type 2 Other Specific Type * Gestational Diabetes ** Normal Glucose Regulation Impaired Glucose Tolerance or Impaired Fasting Glucose (Pre-Diabetes) Diabetes Mellitus Not insulin Insulin requiring Insulin requiring requiring for control for survival Diagnostic Criteria For DM NORMOGLYCEMIA IMPAIRED FASTING GLYCEMIA (IFG) IMPAIRED GLUCOSE TOLERANCE (IGT) DIABETES MELLITUS FPG 2 hours PG (after 75 g OGTT) <<100mg/dL 110 mg/dL (5.6mmol/l) 100mg/dl(5.6mmol) 110 and < 126 mg/dL < 140mg/dL(7.8mmol/l) --- and< 126 mg/dL(7.0) --- 140mg/dl(7.8mmol/l) and < 200 mg/dL(11.1mmol/l) 126 mg/dL(7.0 mmol/l) 200 mg/dL (11.1mmol/l) Symptoms of diabetes and casual plasma glucose of 200 mg/dl(11.1mmol/l) American Diabetes Care Association ADA, Diabetes 2009 2003 Hba1c Integrated summary of circadian blood glucose in the preceding 6-8 weeks Not used as diagnostic test for diabetes Lack of standardized analytical method and therefore lack of a uniform non diabetic reference level between laboratories Insensitive in the low range Normal aic cannot exclude diabetes or IGT Issues on current diagnostic cut off 3 studies on which FPG of 7.0 cutoff was based for diagnosis of diabetes used direct ophthalmoscopic examination and one retinal photograph Diabetes Prevention Program showed substantial prevalence of retinopathy below FPG of 7.0 Cardiovascular complications occur at lower glucose levels Definition and classification of diabetes and pre states should be based on the level of subsequent risk of cardiovascular complications class 1 level B ESC,EASD 2007 Relation between FPG and retinopathy FPG (Mean) Number (%) with Retinopathy Lancet 2008 BMES 5.3 364 (11.5) AusDiab 6.5 210 (9.3) MESA 5.9 959 (15.8) Blue Mountains Eye Study (5-year incident retinopathy) 60 Any retinopathy 50 Percentage 40 30 20 10 0 < 4.6 4.7 - 5.4 5.5 - 6.2 6.3 - 7.0 7.1 - 7.8 7.9 - 8.6 8.7 - 9.4 9.5 - 10.2 > 10.3 Fasting plasma glucose (mmol/L) Number with any retinopathy 40 100 24 12 3 7 3 2 5 Total 545 996 241 56 21 15 9 5 15 Relation between baseline FPG and incident retinopathy, BMES Recommendation Current diagnostic criteria remain the best tools for now. Mechanisms of Glucose Dysregulation and Development of Type 2 Diabetes Genetics 39% of patients with type 2 diabetes have at least one parent with the disease Among monozyzgotic twin pairs with one affected twin, approximately 90% of unaffected twins eventually develop the disease First degree relative of patients with type 2 diabetes frequently have impaired nonoxidative glucose metabolism long before they develop type 2 diabetes Ethnic predilection Environment Low birth weight Gestational diabetes Prematurity Sedentary lifestyle High fat diet Physiologic & Molecular basis of Diabetes Physiologic islet cell dysfunction insulin resistance Molecular insulin receptor Insulin signal transduction Beta-Cell Function Is Abnormal in Type 2 Diabetes A range of functional abnormalities is present Abnormal oscillatory insulin release Increased proinsulin levels Abnormal insulin response Progressive loss of beta-cell functional mass Adapted from Buchanan TA Clin Ther 2003;25(suppl B):B32–B46; Polonsky KS et al N Engl J Med 1988;318:1231–1239; Quddusi S et al Diabetes Care 2003;26:791–798; Porte D Jr, Kahn SE Diabetes 2001;50(suppl 1):S160–S163. First-Phase Insulin Response to IV Glucose Is Lost in Type 2 Diabetes 120 Plasma insulin (µU/mL) Plasma insulin (µU/mL) 120 Normal 100 80 60 40 20 Type 2 Diabetes 100 80 60 40 20 0 0 –30 0 30 60 90 120 Time (min) n=9 normal; n=9 type 2 diabetes. Adapted from Pfeifer MA et al. Am J Med. 1981;70:579–588. –30 0 30 60 90 120 Time (min) Fewer Pancreatic Islets in Type 2 Diabetes Normal Compensation More islets Larger islets More beta cells/islet Nondiabetic Obesity Larger beta cells Type 2 diabetes Decompensation Fewer islets Fewer beta cells/islet Amyloidosis Adapted from Rhodes CJ. Science. 2005;307:380–384. 33 Apoptosis (arbitrary units) Increased Beta-Cell Apoptosis Occurs in Type 2 Diabetes 2.5 * 2.0 1.5 1.0 0.5 0.0 Control Type 2 diabetes *p<0.05. Islet cell death was assessed by an ELISA method, which evaluates the cytoplasmic histone-associated DNA fragments. After incubation absorbance of samples was read spectrophotometrically. Data obtained from pancreatic islets isolated from 6 T2DM organ donors and 10 nondiabetic cadaveric organ donors. Adapted from Marchetti P et al. J Clin Endocrinol Metab. 2004;89:5535–5541. Amylin Amylin co-secreted with insulin Low amylin levels in type 2 diabetes cause or effect is unclear Insulin (µU/ml) 360 330 300 270 240 110 80 150 120 90 60 30 0 Glucagon (µµg/ml) Glucose (mg/100 ml) Insulin and Glucagon Response to a Large Carbohydrate Meal in Type 2 Diabetes 140 130 120 110 100 90 Type 2 diabetes mellitus (n=12)* Nondiabetic controls (n=11) Meal Depressed/delayed insulin response Nonsuppressed glucagon –60 0 60 Time (minutes) *Insulin measured in five patients Adapted from Müller WA et al N Engl J Med 1970;283:109–115. 120 180 240 Incretin Function in Type 2 Diabetes Secretion of GLP-1 impaired Beta-cell sensitivity to GLP-1 decreased Secretion of GIP normal (or slightly impaired) Effect of GIP abolished or grossly impaired Toft-Nielsen M-B et al. J Clin Endocrinol Metab. 2001;86:3717–3723; Kjems LL et al. Diabetes. 2003;52:380–386; Vilsbøll T et al. Diabetologia. 2002;45:1111–1119; Vilsbøll T et al. J Clin Endocrinol Metab. 2003;88:4897–4903. 37 The Pathophysiology of Type 2 Diabetes Includes Islet Cell Dysfunction and Insulin Resistance Glucagon (Alpha cell) Pancreas Insulin (Beta cell) Glucose uptake Glucose output Hyperglycemia Liver Muscle and adipose tissue Buse JB et al. In: Williams Textbook of Endocrinology. 10th ed. Saunders, 2003:1427–1483; Buchanan TA. Clin Ther. 2003;25(suppl B):B32–B46; Powers AC. In: Harrison’s Principles of Internal Medicine. 16th ed. McGraw-Hill, 2005:2152–2180; Rhodes CJ. Science. 2005;307:380–384. Adapted from Kahn CR, Saltiel AR. Joslin’s Diabetes Mellitus. 14th ed. Lippincott Williams & Wilkins; 2005:145–168. Insulin Resistance Genetics Age Weight adipokines Intra-abdominal adiposity is a major contributor to insulin resistance IAA = high risk fat Associated with inflammatory markers Dyslipidaemia (C-reactive protein) Free fatty acids Secretion of adipokines (↓ adiponectin) Insulin resistance DM2 Increased cardiometabolic risk Inflammation IAA: intra-abdominal adiposity Kershaw EE et al, 2004; Lee YH et al, 2005; Boden G et al, 2002 endocannabinoid system dysregulation Weight-dependent Weight Independent Endocannabinoid system Feeding CB-1 blockade Dyslipidaemia Type 2 diabetes Weight Peripheral insulin resistance Hepatic insulin resistance Hepatic glucose output Adiponectin FFA=free fatty acids CETP=cholesterol ester transfer protein Low HDL-C CETP, lipolysis Lipolysis TG-rich VLDL-C FFA Visceral fat Small, dense LDL-C Portal circulation CB-1 blockade Liver CB-1 blockade Modified from: Lam TKT, 2003; Carr DB, 2004; Eckel R, 2005; Pagotto U, 2005; Di Marzo V et al, 2005 Insulin Action decrease in number of insulin receptors any disruption in the transcription transduction of insulin signaling pathway or Summary Glucose metabolism is tightly regulated to maintain desirable glucose levels Glucose dysregulation leads to progressive dysglycemia from prediabetes to frank diabetes The pathophysiology of type 2 diabetes is complex. Involves multiple physiologic and molecular disturbances influenced by multiple genes and environmental factors This offers multiple target sites for therapy and explains the complexity of treatment of DM2