Embryology02-BodyPlanFetalMembranes
advertisement

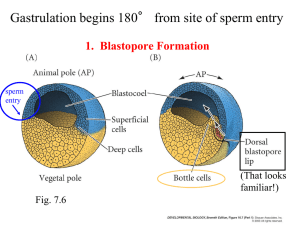
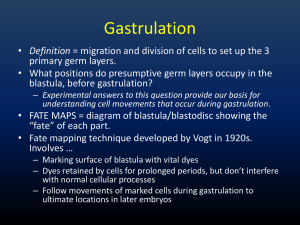
Neurulation, Somitogenesis, General Body Plan, and Fetal Membranes J. Matthew Velkey 454A Davison, Duke South Green Zone matt.velkey@duke.edu Basic structure at 4 weeks The three germ layers are formed at gastrulation Ectoderm: outside, surrounds other layers later in development, generates skin and nervous tissue. Mesoderm: middle layer, generates most of the muscle, blood and connective tissues of the body and placenta. Endoderm: eventually most interior of embryo, generates the epithelial lining and associated glands of the gut, lung, and urogenital tracts. Neural Induction: In addition to patterning the forming mesoderm, the primitive node also sets up the neural plate • • Ectoderm exposed to BMP-4 (from endoderm and mesoderm below), develops into skin However, the node secretes BMP-4 antagonists: (e.g. noggin, chordin, & follistatin) that allow a region of the ectoderm to develop into nerve tissue. Movie: neural induction (click here for QuickTime version) Neurulation: folding of the neural plate 1. Median hinge point forms (probably due to signaling from notochord) –columnar cells adopt triangular morphology (apical actin constriction, like a purse string) 2. Lateral hinge point forms by a similar mechanism (probably due to signaling from nearby mesoderm). 3. As neural folds close, neural crest delaminates and migrates away (more on that later…) 4. Closure happens first in middle of the tube and then zips rostrally and caudally. Neurulation: folding and closure of the neural plate • Folding and closure of the neural tube occurs first in the cervical region. • The neural tube then “zips” up toward the head and toward the tail, leaving two openings which are the anterior and posterior neuropores. • The anterior neuropore closes around day 25. • The posterior neuropore closes around day 28. Movie: Movie: Neurulation Gastrulation to Somitogenesis (click here for QuickTime version) (click here for QuickTime version) Errors in Neurulation: Neural Tube Defects (NTDs) 1. Rachischisis: failure of neural tube folding. 2. Anencephaly: failure of the anterior neuropore closure. 3. Spina bifida: failure of posterior neuropore closure and/or vertebral development. NTDs have many genetic and environmental causes, but strongest correlation is folic acid deficiency. – Best prevented by taking 400 ug folic acid (1000ug if family history of NTDs) daily 3 months prior to conception and throughout pregnancy. Rachischisis: failure of neurulation; i.e., the neural tube does not close Rachischisis: spinal cord Cranioschisis: brain Craniorachischisis: brain & cord Failure of neuropores to close can cause neural tube defects anterior neuropore: anencephaly posterior neuropore: spina bifida Anencephaly: failure of anterior neuropore closure Neural tube closure defects A. Rachischisis, B. Spina bifida occulta, C. Meningocele, D. Myelomeningocele Spina bifida is often observed in the lumbar region reflects to the approximate original location of the posterior neuropore “Regression” of the spinal cord The spinal cord and the vertebral column are the same length up until the 3rd month. As each vertebral body grows thicker, the overall length of the vertebral column begins to exceed that of the spinal cord such that , in the adult the spinal cord terminates at L2 or 3 and the dural sac ends at about S2. The tail end of the dural sac covering the spinal cord and nerve roots remains attached at the coccyx and becomes a long, thin strand called the filum terminale. Sometimes, the spinal cord can become “tethered” or attached to the dural sac or filum terminale; this pulls on the cord and can obstruct flow of CSF thus causing swelling of the ventricles of thebrain (hydrocephalus), Hydrocephalus due to a tethered spinal cord Disrupting flow of CSF causes it to accumulate within the ventricles of the brain –increased pressure leads to swelling of the entire cranium. Neural Crest: the th “4 germ layer” At the time of neurulation, cells at the lateralmost edge of the neural plate are exposed to a unique combination of factors from the adjacent skin, underlying mesoderm, and from the rest of the neural plate and are induced to form neural crest. The neural crest cells downregulate cadherin expression and delmainte from the neuroepithelium, i.e., they transform from epithelial cells into migratory mesenchymal cells that contribute to forming MANY tissues in the body. Major Derivatives of the Neural Crest Mesoderm is patterned in a cranial to caudal gradient Axial mesoderm: passes through the node and migrates along the midline –forms the notochord Paraxial mesoderm: passes just caudal to the node and migrates slightly laterally –forms cartilage, skeletal muscle, and dermis Lateral plate mesoderm: passes more caudal and migrates more laterally – forms circulatory system and body cavity linings. Extraembryonic mesoderm: passes most caudal and migrates most laterally –forms extraembryonic membranes and associated connective tissue & blood vessels. Subdivision of the mesoderm Chordamesoderm – notochord Paraxial mesoderm – head mesenchyme – somites • sclerotome • myotome • dermatome Intermediate mesoderm – urogenital organs Lateral plate mesoderm – splanchnic (viscera) – somatic (body wall) – extraembryonic Somitogenesis Paraxial mesoderm form pairs of somitomeres (pre-somites) Somitogenesis begins w/ 8th pair of somitomeres (pairs 1-7 don’t develop into somites) Mesoderm (mesenchyme) is transformed back into epithelium –relies on Noggin antagonism of BMP-4 Noggin • • • BMP Separation of Somites • • FGF signaling from the node drives proliferation; Retinoic acid from adjacent mesoderm drives differentiation. Because the node is caudal to the forming somites, there is a head-to-tail gradient of differentiation. Proliferating cells express a ligand (called ephrin B1). As cells differentiate, they begin to express an INCOMPATIBLE receptor (called eph A) that causes the differentiating cells to repel the proliferating cells, thus pinching off and forming a new somite. N-cad (click here for QuickTime version) Somites are balls of epithelial cells with a few mesenchymal cells in the core Epithelial somites then transform back into mesenchyme • Signaling from ectoderm induces dermomyotome • Signaling from notochord and neural tube induces sclerotome Dermamyotome forms dermis and muscle • BMP from ectoderm induces dermatome the remaining dorsomedial and ventrolateral cells become myotome. • Dorsomedial portion of dermomyotome sees Shh from notochord and Wnt from spinal cord and becomes muscle that can’t migrate very far (epaxial muscles of the back) • Ventrolateral portion of dermomyotome exposed to high levels of BMP from lateral plate mesoderm and becomes migratory muscle (goes into limbs also “hypaxial” muscles of the lateral and ventral body wall, e.g. “lats” and “abs” The sclerotome develops into vertebrae, ribs, and meninges Dorsal sclerotome: – Dorsal arch & spinous processes of vertebrae* Medial sclerotome: – Meninges* Central sclerotome: – Pedicles & transverse processes of vertebrae, proximal portions of ribs Ventral sclerotome: – Vertebral bodies and annulus fibrosis of intervertebral disks Lateral sclerotome: – Distal portions of ribs *failure of these associated w/ spina bifida Somite (Paraxial mesoderm) forms: 1. Sclerotome 2. Dermamyotome Sclerotome forms: Meninges, vertebral bodies & ribs Dermamyotome forms: 1. Dermis (from dermatome) 2. Muscles (from myotome) (click here for QuickTime version) The sclerotome breaks into two parts… Anterior and posterior portions of each sclerotome fuse to form vertebral bodies. This offsets the vertebral bodies and segmental muscles. i.e., the vertebrae (sclerotome) are out of phase with muscle (myotome) to form intervertebral joints. This allows the contracting segmental muscles to move the vertebral column laterally. Anterior portion of one somite fuses with the posterior portion of the next somite; that way The surrounding muscle bridges sequential vertebral bodies. (click here for QuickTime version) Intermediate mesoderm develops into the urogenital system Lateral plate divides into somatic and splanchnic mesoderm Somatic mesoderm: Lines body wall (somatic mesoderm + ectoderm = somatopleure) Splanchnic mesoderm: Covers endoderm (splanchnic mesoderm + endoderm = splanchnopleure) Coelom = body cavity formed by lateral folding of the embryo Lateral plate mesoderm also generates extraembryonic mesoderm of amnion, yolk sac, and placenta. Somatic (aka parietal) mesoderm stays with epidermis Splanchnic (aka visceral) mesoderm stays with endoderm Blood and blood vessels develop from extraembryonic and lateral plate mesoderm • • • • Vasculogenesis: blood vessels arise de novo from “hemangioblasts” that develop into blood cells AND vascular tubes Angiogenesis: growth of new blood vessels from existing ones Vessels from extraembryonic mesoderm go out to placenta and eventually hook up with blood vessels (and the heart) in the embryo that arise from lateral plate mesoderm to establish circulation. 2 major phases of hematopoiesis: – – Embryonic (weeks 1-4) : blood cells arise from yolk sac mesoderm Definitive (week 4-term): blood cells arise from lateral plate mesoderm in the AGM (aorta-gonad-mesonephros region) that go on to seed the spleen, liver, and then bone marrow with hematopoietic stem cells. Segmentation of the endoderm Pharyngeal Gut Oropharyngeal membrane (stomodeum) to pharynx Foregut Trachea, esophagus, stomach, duodenum, liver, and pancreas Midgut Small intestine, ascending colon, proximal 2/3 of transverse colon Hindgut Distal 1/3 of transverse colon, descending colon, rectum, cloacal plate (proctodeum) Segmentation of the endoderm: movie (click here for QuickTime version) (click here for QuickTime version) Closure of the body wall Craniocaudal and lateral folding draw in the yolk sac (like a pursestring) and close off the body wall except at the umbilicus. Simultaneous events: Purse string-like closure of the body wall Growth of the amnion, Regression of yolk sac. Embryo grows into the amnion. Yolk sac regresses. (click here for QuickTime version) Ectopia cordis: failure of the thoracic body wall to close Gastroschisis: failure of the abdominal body wall to close Implantation & Placentation The placenta is derived from the trophoblast cells that further differentiate and invade maternal tissues – Cytotrophoblast: stem cell population – Syncytiotrophoblast: invasive fused cells (syncytium) derived from cytotrophoblast – Invasion process breaks into maternal capillaries, trophoblastic lacunae fill with maternal blood (click here for QuickTime version) The placenta allows for exchange (NOT mixing) between maternal and fetal blood • Early placental barrier (weeks 1-12) has more layers: – – – – Syncytium Cytotrophoblast Embryonic conn. tissue Endothelium • Later placental barrier (4th mo. – term) has fewer layers: early later – Syncytium – Endothelium Summary of fetal membranes (and cavities) • • • Chorion: (part of the placenta) ring of extraembryonic mesoderm from which placental villi sprout Chorionic cavity: aka extraembryonic coelom, space between chorion and developing embryo (gets smaller as the embryo and amnion expand) Amniotic cavity: – – • • space that is initially on the dorsal surface of the embryo and then almost completely envelops embryo with folding and closure of the body wall bounded by the amniotic membrane and filled with amniotic fluid Yolk sac: space that is on the ventral surface of the embryo and is drawn closed (like a pursestring) with closure of the body wall. Umbilical cord: – – – – connection from the embryo to the chorion/placenta contains arteries conveying nutrient and oxygenpoor blood to the placenta and a single vein conveying enriched blood back to the fetus Also contains closed off yolk sac During gut development intestinal loops herniate into the umbilical cord and are eventually drawn back into the fetus Fertilization to term: the movie (click here for QuickTime version) Amniotic fluid Typical volumes: 30 ml @ 10 wks; 450 ml @ 20 wks; 1l @ 37 wks Produced by: amnion, maternal blood, fetal kidneys (5th month) Taken up by fetal swallowing Polyhydramnios: > 1500 ml (often result of gut or swallowing defect, also secondary to maternal diabetes) Oligohydramnios: < 400 ml failure to produce enough fluid (usu. Renal defects) Amniotic Bands: The amnion can ensnare parts of the fetus (usu. limbs) and cause constriction or even amputation. Often associated with oligohydramnios. Twinning may result in sharing of placenta and/or fetal membranes Shared placenta may result in twin transfusion syndrome where more blood goes to one fetus. Dizygotic (aka “paternal”): Monozygotic (aka “maternal”):