Laboratory Information Management Systems
advertisement

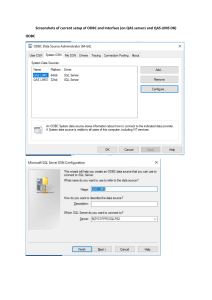
www.GlobalHealthLaboratories.org This teaching material has been made freely available by the KEMRI-Wellcome Trust (Kilifi, Kenya). You can freely download, adapt, and distribute this presentation under the conditions that: the Global Health Laboratories and The Global Health Network are referenced; the work is not used for commercial purposes, and any altered forms of this document are distributed freely under the same conditions. Laboratory Information Management Systems (LIMS) Good Clinical Laboratory Practice KEMRI-Wellcome Trust Research Programme What is LIMS? Computerized information management system designed for laboratories Manages lab data from sample log-in to reporting Interfaces with analytical instruments Sorts and organizes data into various report formats Stores data for future reference and use Rationale for LIMS hardware, software, people, procedures and data Role of LIMS To manage Data Work flow Changing business needs/processes Existing systems and improving where required Resources QA/QC Why have a LIMS? Improve data management in lab to increase lab potential Enable centralization of information Support and enhance business processes of the lab Take advantage of new lab information technology Provide easy access to data Functions of LIMS Track specimens from receipt, processing, testing, reporting to storage Electronically capture results from lab diagnostic equipment and store with specimen details Protocols and algorithms for testing and final result determination Functions of LIMS Patient focus Enable determination of patient outcomes Integrate patient and specimen information Support patient management and care/treatment LIMS decision Type of lab Reference/research/public health Clinical Hybrid Volume of specimens Types and number of tests Size of staff/users Existing system Determine which areas will be affected Requirements and expectations Avoid ‘culture shock’ Advantages of LIMS Use Fewer transcription errors & faster processing with direct instrument uploads Real time control of data quality with built in QC criteria Direct report generation meeting specific client requirements Direct electronic reporting to clients or direct client access to data Disadvantages/Concerns Customization of LIMS/interfaces required for specific lab/client needs Adequate validation to ensure data quality Data integrity and confidentiality, especially when clients have direct access to data Limited interface between lab & field computer systems Examples KIDMS Reports Information available on LIMS Project, samples, tests documentation Sample tracking history within the Lab Reporting results (hardcopy, electronic file) Financial information by test, client, dates Information on Productivity Benefits of LIMS It improves the efficiency hence productivity. Improve data quality (all instrument are integrated). Saves time because the information is obtained at the snap of the button Improve level of data access for all stakeholders of any project. Automated customer reports (TAT, Work Load) Laboratory Responsibilities Evaluate LIMS capabilities System validation/maintenance Interfacing capabilities Confidentiality/data integrity protection Regulatory compliance/accreditation.