This Presentation - School of Biomedical Engineering
advertisement
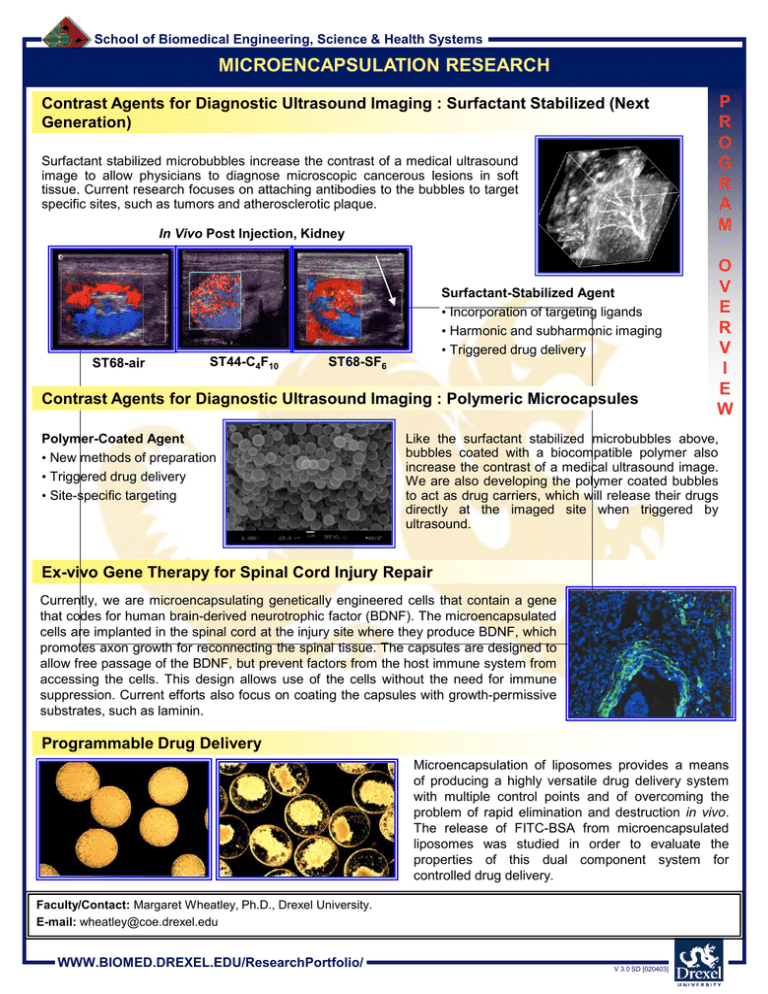
School of Biomedical Engineering, Science & Health Systems MICROENCAPSULATION RESEARCH Contrast Agents for Diagnostic Ultrasound Imaging : Surfactant Stabilized (Next Generation) Surfactant stabilized microbubbles increase the contrast of a medical ultrasound image to allow physicians to diagnose microscopic cancerous lesions in soft tissue. Current research focuses on attaching antibodies to the bubbles to target specific sites, such as tumors and atherosclerotic plaque. In Vivo Post Injection, Kidney ST68-air ST44-C4F10 Surfactant-Stabilized Agent • Incorporation of targeting ligands • Harmonic and subharmonic imaging • Triggered drug delivery ST68-SF6 Contrast Agents for Diagnostic Ultrasound Imaging : Polymeric Microcapsules Polymer-Coated Agent • New methods of preparation • Triggered drug delivery • Site-specific targeting P R O G R A M O V E R V I E W Like the surfactant stabilized microbubbles above, bubbles coated with a biocompatible polymer also increase the contrast of a medical ultrasound image. We are also developing the polymer coated bubbles to act as drug carriers, which will release their drugs directly at the imaged site when triggered by ultrasound. Ex-vivo Gene Therapy for Spinal Cord Injury Repair Currently, we are microencapsulating genetically engineered cells that contain a gene that codes for human brain-derived neurotrophic factor (BDNF). The microencapsulated cells are implanted in the spinal cord at the injury site where they produce BDNF, which promotes axon growth for reconnecting the spinal tissue. The capsules are designed to allow free passage of the BDNF, but prevent factors from the host immune system from accessing the cells. This design allows use of the cells without the need for immune suppression. Current efforts also focus on coating the capsules with growth-permissive substrates, such as laminin. Programmable Drug Delivery Microencapsulation of liposomes provides a means of producing a highly versatile drug delivery system with multiple control points and of overcoming the problem of rapid elimination and destruction in vivo. The release of FITC-BSA from microencapsulated liposomes was studied in order to evaluate the properties of this dual component system for controlled drug delivery. Faculty/Contact: Margaret Wheatley, Ph.D., Drexel University. E-mail: wheatley@coe.drexel.edu WWW.BIOMED.DREXEL.EDU/ResearchPortfolio/ V 3.0 SD [020403] School of Biomedical Engineering, Science & Health Systems CONTRAST AGENTS FOR DIAGNOSTIC ULTRASOUND IMAGING: SURFACTANT STABILIZED (NEXT GENERATION) Surfactant stabilized microbubbles increase the contrast of a medical ultrasound image to allow physicians to diagnose microscopic cancerous lesions in soft tissue. Current research focuses on attaching antibodies to the bubbles to target specific sites, such as tumors and atherosclerotic plaque. P R O J E C T In Vivo Pre Injection ST68-air ST44-C4F10 ST68-SF6 O N E Color Doppler Imaging: ST68-PFC and ST44-PFC, ~1 x 10*9 microbubbles/mL Injected in right jugular vein of NZ white rabbit (Siemens Elegra Imaging, right kidney) In Vivo Post Injection P A G E R 3-D Harmonic Image of Kidney Surfactant-Stabilized Agent • Incorporation of targeting ligands • Harmonic and subharmonic imaging • Triggered drug delivery In vivo Harmonic Imaging ST44-PFC Pre-injection MI 0.3 Post-injection of 0.1 ml/kg ST68-air ST44-C4F10 ST68-SF6 Color Doppler Imaging Pre-injection Post-injection CAI ST68 0.1 mL/Kg ; MI 0.4, 3.3/6.6 MHz (2nd) Faculty/Contact: Margaret Wheatley, Ph.D., Drexel University. E-mail: wheatley@coe.drexel.edu Collaborating Researchers: F. Forsberg, Ph.D., Thomas Jefferson University; B. Goldberg, Ph.D., Thomas Jefferson University. Funding: NIH Laboratories: Calhoun Laboratories at Drexel University. WWW.BIOMED.DREXEL.EDU/ResearchPortfolio/ V 3.0 SD [020403] School of Biomedical Engineering, Science & Health Systems CONTRAST AGENTS FOR DIAGNOSTIC ULTRASOUND IMAGING: POLYMERIC MICROCAPSULES Bubbles coated with a biocompatible polymer increase the contrast of a medical ultrasound image to allow physicians to diagnose microscopic cancerous lesions in soft tissue. P R O J E C T In Vivo Power Doppler Imaging Pre-injection Scanning elecron micrograph of capsules Post-injection Drug Delivery (Therapeutic) Contrast Agents We are also developing the polymer coated bubbles to act as drug carriers, which will release their drugs directly at the imaged site when triggered by ultrasound. Protein Released with Time Protein Released (mg) 2.19 Stirred 1.99 F=5MHz and P=2.07 MPa 1.79 O N E P A G E R F=10MHz and P=1.25 MPa 1.59 F=10MHz and P=2.63 MPa 1.39 F=5MHz and P=0.838 MPa 1.19 0 Time (min) 5 10 15 Targeted Contrast Agents Current research focuses on attaching antibodies to the bubbles to target specific sites, such as tumors and atherosclerotic plaque. Attachment of PLGA Contrast Agents to Cells after 6 Hours of Incubation Control 1- Cells without capsules Control 2 - PLGA capsules attached without peptide Experimental- PLGA capsules attached with peptide Faculty/Contact: Margaret Wheatley, Ph.D., Drexel University. E-mail: wheatley@coe.drexel.edu Collaborating Researchers: F. Forsberg, Ph.D., Thomas Jefferson University; B. Goldberg, Ph.D., Thomas Jefferson University. Funding: NIH Laboratories: Calhoun Laboratories at Drexel University. WWW.BIOMED.DREXEL.EDU/ResearchPortfolio/ V 3.0 SD [020403] School of Biomedical Engineering, Science & Health Systems EX-VIVO GENE THERAPY FOR SPINAL CORD INJURY REPAIR Currently, we are microencapsulating genetically engineered cells that contain a gene that codes for human brain-derived neurotrophic factor (BDNF). The microencapsulated cells are implanted in the spinal cord at the injury site where they produce BDNF, which promotes axon growth for reconnecting the spinal tissue. The capsules are designed to allow free passage of the BDNF, but prevent factors from the host immune system from accessing the cells. This design allows use of the cells without the need for immune suppression. Current efforts also focus on coating the capsules with growthpermissive substrates, such as laminin. In vitro cell growth in microcapsules Transplantation of encapsulated cells in vivo a b a a. Microcapsules inside the spinal cord of a rat. b Cells encapsulated at high concentration a b. BDNF producing fibroblasts encapsulated with cresol violet inside the microcapsules. P R O J E C T O N E P A G E R Day 3 Day 5 Section of spinal cord stained with RT97, which stains neurofilament, a cytoskeletal protein of neurons. The red color shows growth of neurons around the microcapsule. Day 7 Section of spinal cord stained with CGRP, which stains a neurotransmitter. The green color provides evidence of sprouting of neurons. Day 12 At day 5, some of the capsules were broken open by treatment with EDTA, and the released cells were allowed to grow in culture Immediately after release from capsules 2 days post-release Cross-section of implant area: the green stain represents neuronal growth on the periphery of the implanted capsule (the arrow indicates the genetically engineered cell aggregate inside the capsule). Faculty/Contact: Margaret Wheatley, Ph.D., Drexel University. E-mail: wheatley@coe.drexel.edu Collaborating Researchers: I. Fischer, Ph.D., MCP Hahnemann University; M. Murray, Ph.D., MCP Hahnemann University. Funding: NIH Laboratories: Calhoun Laboratories at Drexel University. WWW.BIOMED.DREXEL.EDU/ResearchPortfolio/ V 3.0 SD [020403] School of Biomedical Engineering, Science & Health Systems PROGRAMMABLE DRUG DELIVERY Microencapsulation of liposomes provides a means of producing a highly versatile drug delivery system with multiple control points and of overcoming the problem of rapid elimination and destruction in vivo. The release of FITC-BSA from microencapsulated liposomes was studied in order to evaluate the properties of this dual component system for controlled drug delivery. Alginate was used as the encapsulating polymer due to the mild conditions of the encapsulation process. Liposomes composed of phosphatidylcholine (PC) and cholesterol (Chol) in the molar ratio 7:3 and of phosphatidylcholine, phosphatidylglycerol (PG) and cholesterol in the ratio 6:1:3 were encapsulated in alginate crosslinked with Ca2+ (Ca-Alg), Al3+ (Al-Alg) and Ba2+ (Ba-Alg). A rapid initial burst of protein release was observed from liposomes that were encapsulated in Ca-Alg and Al-Alg. The extent of the burst was slightly different in liposomes encapsulated in Ca-Alg and Al-Alg and no burst was observed in those encapsulated in Ba-Alg indicating that the crosslinking ions could significantly affect the release of entrapped protein. Also, release of FITC-BSA from liposomes encapsulated in CaAlg varied significantly with liposome composition. Release studies indicated that cholesterol containing liposomes were leakier after encapsulation in Ca-Alg compared to those without cholesterol. In all cases, the release from microencapsulated liposomes was much faster than that from free liposomes suggesting an interaction between the liposomes and the alginate. Differential scanning calorimetry suggested that alginate was inserted into the lipid bilayer resulting in a rapid release of protein from microencapsulated liposomes. Also, it was observed that the degree of interaction between liposomes and alginate had a strong dependence on liposome composition. Immediately after encapsulation Ten days after start of delivery Alginate-encapsulated liposomes. Over time, the lipsomes discharge the drug. Sustained release can last up to 30 days. Faculty/Contact: Margaret Wheatley, Ph.D., Drexel University. E-mail: wheatley@coe.drexel.edu Collaborating Researchers: H. Shaw, Ph.D., NASA. Funding: NASA Laboratories: Calhoun Laboratories at Drexel University. WWW.BIOMED.DREXEL.EDU/ResearchPortfolio/ V 3.0 SD [020403] P R O J E C T O N E P A G E R





![Jiye Jin-2014[1].3.17](http://s2.studylib.net/store/data/005485437_1-38483f116d2f44a767f9ba4fa894c894-300x300.png)





