Adam Sowatzka - CAA 112(r) Federal Program Update
Hot Topics in CAA – 112(r)
Federal Program Update
GA AWMA REGULATORY
UPDATE CONFERENCE
Adam G. Sowatzka
April 16, 2013
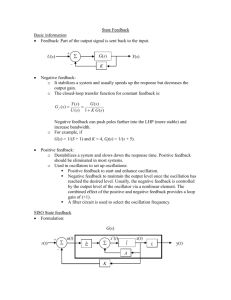
Agenda
• Background
• Risk Management Program
• General Duty Clause
• EPA Enforcement
• Questions
2
Background
3
Why Address Risk Management?
• Congressional response to preventing further major chemical accidents:
― Bhopal, India (Methyl
Isocyanate release, 2,800 deaths, 1984)
― Institute, West Virginia
(100 plus injured, 1985)
― Pasadena, Texas (plastics plant fire, 23 deaths, 1989) http://www.youtube.com/watch?feature=player_detailpage&v=3l2PQEjMnnM
4
Risk Management Program –
Federal Statutory Provisions
• EPCRA – Emergency Planning and Community
Right-to-Know Act (SARA Title III), Sections 302-
312
• CERCLA – Comprehensive Environmental
Response, Compensation and Liability Act, Section
103
• CAA – Clean Air Act Section 112(r)(7), Chemical
Accident Prevention Provisions and Risk
Management Plans; and Section 112(r)(1) General
Duty Clause
5
CAA Section 112(r)
• Regulatory requirement for subject facilities to file a Risk Management Plan (RMP) by June 21,
1999 or before covered chemical is on site
•
Includes “General Duty Clause” (GDC) requiring facilities to consider hazards and minimize risk posed by chemicals
6
EPA’s Risk Management Program
7
Who is subject to the RMP regulations?
• Stationary sources that have more than a threshold quantity (TQ) of a listed substance
• EPA has developed a list of chemicals:
― 77 toxic
― 63 flammable
• If a facility stores one of these chemicals at quantities greater than a TQ….. THE FACILITY
MUST HAVE A RISK MANAGEMENT PLAN
8
Key Elements of RMP
• Employee participation plan
• Process safety information (documentation of the process)
• Process Hazard Analysis (PHA)
• Operating procedures
• Operator training
• Contractor evaluation and selection
• Pre-start-up safety reviews
9
Key Elements of RMP Cont’d
• Mechanical integrity program
• Hot work permitting process
• Management of Change (MOC)
• Incident investigation
• Emergency planning and response
• Compliance audits
10
RMP Filing
• Facilities must resubmit RMPs at 5 year intervals
• There are additional/on-going responsibilities − it is not a static program that ends with filing of RMP
• Certification of receipt and completion from RMP
Reporting Center does not indicate that an RMP is in compliance with regulations
11
Important Dates Reported in RMPs
• Process Hazard Analysis
(PHAs), compliance audits, and SOP review dates
• Red flags:
― Leaving these entries blank or having future dates
― Having dates (as of the filing) that are more than 5 years, 3 years, and 1 year past due
12
PHAs and Compliance Audits
• Must be performed by proper personnel
• Corrective actions/recommendations required for noted deficiencies
• If completion dates are not shown in facility documentation, objective quality evidence is required
13
General Duty Clause
14
General Duty Clause – CAA §112(r)(1)
• Statutory requirement, effective as of November
1990
• No list of covered substances, no threshold quantities
• No reporting requirement, information sharing with public not required
• No exemptions or exclusions
15
General Duty Clause – CAA §112(r)(1)
• The owners and operators of stationary sources producing, processing, handling or storing such substances [i.e., a chemical in 40 CFR part 68 or any other extremely hazardous substance] have a general duty [in the same manner and to the same extent as the general duty clause in the Occupational Safety and Health Act (OSHA)] to identify hazards which may result from (such) releases using appropriate hazard assessment techniques, to design and maintain a safe facility taking such steps as are necessary to prevent releases, and to minimize the consequences of accidental releases which do occur.
16
Substances Covered Under GDC
• Extremely hazardous substances
― Short-term exposures associated with releases to air may cause death, injury, or property damage due to toxicity, reactivity, flammability, volatility, or corrosivity
• Includes, but not limited to, RMP list of toxic and flammable substances
17
Facility Responsibilities Under GDC
• Identify hazards of chemicals, and assess impact of potential releases
• Design and maintain safe facilities
• Follow codes, standards, and other business practices
• Minimize consequences of accidental releases
18
GDC – Considerations for Safe Practices
• What are similar businesses doing to minimize hazard?
― Codes and standard practices
― EPA and other Safety Alerts, Case Studies, and
Investigation Reports
― Trade association guidelines
• What is the accident history of my industrial sector?
― Lessons learned
19
EPA Enforcement
20
EPA’s Enforcement Initiatives
Fiscal Years 2011-2013:
• Preventing the release of raw sewage and contaminated stormwater
• Preventing animal waste from contaminating surface and ground waters
• Cutting toxic air pollution that affects health
• Reducing air pollution from largest sources
• Reducing pollution from mineral processing operations
• Assuring energy extraction sector compliance
21
•
EPA Enforcement Overview
Inspection
• Information request
• Administrative Compliance
Order
• Penalty action
― Administrative
― Judicial Referral
― Criminal
22
EPA RMP and GDC Enforcement
Case
Tyson Foods
JP Lillis Enterprises, d/b/a Cape Cod Ice
Suiza Dairy
Corporation
C.A.I., Inc. of
Danvers,
Massachusetts
BP Products North
America Inc.
D.D. Williamson &
Co, Inc.
Date Penalty Injunctive Relief
4/4/2013 $3.9 million RMP audits at 21 facilities
1/9/2013 $225,000
9/28/2012 $275,000
Various corrective actions related to its ammonia program
$3.75 million in facility upgrades
8/15/2011 $100,000 $1.3 million for site clean-up related to a removal action caused by a fire
9/30/2010 $15 million Implement defined compliance program including monthly reports to EPA
10/7/2009 $300,000 Conduct RMP audit and take corrective actions based on that audit
23
Questions
Adam G. Sowatzka
Partner
King & Spalding
1180 Peachtree Street, N.E.
Atlanta, GA 30309-3521
Direct: 404-572-3508
Fax: 404-572-5136
Cell: 770-309-5349 asowatzka@kslaw.com
www.kslaw.com
24