January 14, 2015 Research Updates Presentation
advertisement

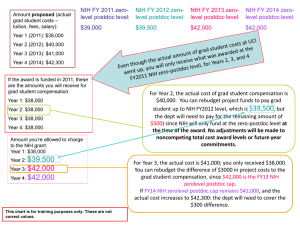
ORSP Research Updates January 14, 2015 10:30 am IOP Auditorium Topics Covered • • • • Uniform Guidance Update NIH Notices & Updates Process Reminders Miscellaneous Updates Uniform Guidance Update • Uniform Guidance – Effective 12/26/2014 – NIH Plans • 12/19/2014 HHS adopted 2 CFR part 200 http://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-046.html • INTERIM Terms and Conditions (coming soon) • UPDATED Grants Policy Statement (replaces 10/1/13 GPS) – STAY TUNED for more updates… Uniform Guidance Update • Uniform Guidance – NEARLY all FDP federal agencies working to have standard Terms and Conditions • Currently DOD agencies are NOT participating – FDP federal agencies will update Prior Approval Matrix based on UG • TWO agencies agree discussion – INPUT from Grantee Community will be considered • COGR and FDP will mostly likely lead these input efforts NIH Notices NOT-OD-15-039 • 2 week Window of Consideration for Late Application Submission • Acceptance of late applications will be made on a case-by-case basis, dependent upon the explanation provided in a cover letter submitted with the application • NIH does not expect to accept any applications received beyond the window of consideration or for RFAs that specify no late applications will be accepted. • Reasons for late submission must be in relation to the individual(s) with the PD/PI role on the application • For multiple PD/PI (MPI) applications, the reasons may apply to any or all of the PD/PIs NIH Notices Examples of Reasons Why Late Applications Might Be Accepted • Death of an immediate family member of the PD/PI (or MPI). • Sudden acute severe illness of the PD/PI (MPI) or immediate family member. • Temporary or ad hoc service by a PD/PI on an NIH advisory group during the two months preceding or the two months following the application due date. Examples of qualifying service include: participation in an NIH study section/special emphasis panel, NIH Board of Scientific Counselors, Program Advisory Committee, or an NIH Advisory Board/Council. Qualifying service does not include participation in NIH activities other than those involved in extramural/intramural peer review or NIH Advisory Council/Board service. • Delays due to weather, natural disasters, or other emergency situations, not to exceed the time the applicant organization is closed. • For PD/PIs who are eligible for continuous submission (http://grants.nih.gov/grants/peer/continuous_submission.htm), the late application policy applies to activities not covered under the continuous submission policy (i.e., other than R01, R21, and R34 funding opportunities that use standard due dates). NIH Notices Examples of Reasons Why Late Applications Will Not Be Accepted • Heavy teaching or administrative responsibilities, relocation of a laboratory, ongoing or non-severe health problems, personal events, participation in review activities for other Federal agencies or private organizations, attendance at scientific meetings, or a very busy schedule. • Review service for participants other than a PD/PI or MPI, acute health issues or death in the family of a participant other than a PD/PI or MPI. • Problems with computer systems at the applicant organization, problems with a system-to-system grant submission service, or failure to complete or renew required registrations in advance of the application due date. • Failure to follow instructions in the Application Guide or funding opportunity announcement. • Correction of errors or addressing warnings after 5 PM local (applicant organization) time on the application due date. Applicants are encouraged to submit in advance of the due date to allow time to correct errors and/or address warnings identified in the NIH validation process. NIH Notices • NOT-OD-15-044 – ASSIST To Become an Option for Submission of Applications for Most Competing Grant Programs in 2015 – Throughout 2015, ASSIST will become an option on a grant program by grant program basis (by activity code). The first set of grant programs will include the R03 and R21 programs and is targeted for the end of January 2015. – Use of ASSIST is optional; Grants.gov downloadable forms and system-to-system solutions also remain viable options for application preparation and submission to NIH – NOTE: Cayuse is more user friendly and has additional functionality NIH Notices NOT-OD-15-048 • FY 2015 Kirchstein-NRSA Budgetary Levels • All FY 2015 awards previously issued using FY 2014 stipend levels will be revised to adjust stipends to the FY 2015 level. • Appointments to institutional training grants that have already been awarded in FY 2015 must be amended via xTrain to reflect the FY 2015 stipend levels once the training grant award has been adjusted by the NIH • Current stipend levels are to be used in the preparation of future competing and non-competing NRSA institutional training grant and individual fellowship applications. They will be administratively applied to all applications currently in the review process. • The NIH will provide funds for Tuition and Fees, Training Related Expenses, and Institutional Allowance, as detailed below. Those amounts do not change with the implementation of the FY 2015 budget. NIH Notices NOT-OD-15-049 • FY 2015 NIH salary cap is $183,300 effective January 11, 2015 • Salaries up to this level are allowed to be charged starting January 11, 2015, to NIH funded grants provided the necessary funds are available. • The ORSP Proposal Preparation page and Cayuse data have been updated to reflect the new NIH Salary Cap level. • Please use the new salary cap maximum when preparing all future NIH grant proposals. NIH Notices • NOT-OD-15-051 – NIH Requirement for Federal Recognition of Same-Sex Spouses/Marriages by Grant and Research and Development Contract Recipients – Marital or spousal considerations are relevant to the NIH grants and R&D contracts communities in the collection of financial conflicts of interest (COI) information • 42 C.F.R. Part 50, Subpart F, Promoting Objectivity in Research • 45 C.F.R. Part 94, Responsible Prospective Contractors • 42 C.F.R. Part 52h, Scientific Peer Review of Research Grant Applications and Research and Development Contract Projects Process Reminders • INTERNAL to MUSC – Congruency Review Process Change • ALL PHS Just-In-Time (JIT) AFTER 1/1/2015 • & ALL PHS Awards (excludes non-competes) AFTER 3/1/15 • More Information – SEE November 18th slide presentation http://academicdepartments.musc.edu/research/orsp/ – Confidential Disclosure Agreements (CDA’s) • Reviewed, Approved & Signed by ORSP • ? about CDA’s call the ORSP Grants Administrator – ePDS (routed & approved)BEFORE agreement review can begin in ORSP Process Reminders • EXTERNAL to MUSC – DOD 2nd level approval for MUSC approved protocols and amendments (both humans and animals) • MANDATORY for DOD, different than NIH • Anticipate DELAYS and impact on MUSC project • NONCOMPLIANCE will be EXPENSIVE! – ClinicalTrials.gov https://clinicaltrials.gov/ • Portal to be Improved: “The Carrot Approach” • More Focus on Compliance • Noncompliance RISKS ALL MUSC’s federal funding “The BIG Stick Approach” Miscellaneous Updates • INTERNAL – F&A rates determined by ACTIVITY and LOCATION • SPONSOR does NOT dictate MUSC’s F&A rate • A lot of sub-awards does NOT dictate OFF campus • Questions, PLEASE call ORSP Grants Administrator http://academicdepartments.musc.edu/research/orsp/proposal_preparation/#rates – Training Sessions and Dates Planned for 2015 http://academicdepartments.musc.edu/research/orsp/training/ • EXTERNAL – Grants.gov PLANNED shutdown Feb. 14-17 – NSF to roll-out BUILT-IN proposal submission compliance checks (end of January 2015) • MUST be 100% compliant to be reviewed • PI’s WILL get system generated error messages THANK YOU • Questions/Comments? • PLEASE complete a survey & THANK YOU • Next ORSP Information Session: February 18, 2015 @ 10:30 am, IOP Auditorium






![NIH 101: Part 1 [.ppt]](http://s2.studylib.net/store/data/005398706_1-cbe361c448786ac362a8e75ad39fc05d-300x300.png)