SCCA Monitors
advertisement

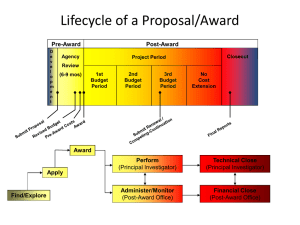
Tips to a Successful Monitoring Visit Ngoc Tran, CCRA NT Research, Inc. Study Monitor Person delegated by the Sponsor to oversee the conduct of a clinical trial by ensuring that study activities are in accordance to the protocol and regulatory requirements. Roles and Responsibilities Act as a liaison between Site and Sponsor Assure rights, safety and well-being of subjects are protected Review trial data for accuracy, completeness and verifiable Oversee study activities for protocol, GCP and regulatory compliance Different but Same Changing mindsets All for One and One for All Monitor-Site Encounters Site Visits Evaluation/Pre-Study Initiation Monitoring Close-out Assess Investigator’s interest and qualification Evaluate Site’s capability to properly and safely conduct clinical trial Request Investigator and Study Coordinator attendance Evaluation/ Pre-Study Visit Purpose Setting the stage. Obtain Signed Confidentiality Agreement Provide protocol synopsis for review Evaluation/ Pre-Study Visit Expectations Meet & Greet Evaluate by phone or in person Preparing for an Evaluation/Pre-Study Visit Review protocol synopsis Obtain copies of the Investigator’s CV and credentials Verify that there is sufficient personnel, resources and necessary equipment to conduct the clinical trial Assess ability to meet enrollment goals and timelines Schedule tour of facilities (i.e. pharmacy, lab, etc). Provide Protocol and Investigational Product/Device Training Outline expectations of Investigator and research personnel Confirm study supplies received Initiation Visit Purpose Why Initiations are so critical for the Sponsor and the Site to engage in open dialogue. Collect remaining essential documents Occurs prior to enrollment commencing Attendance expected of Investigator and Key Research Personnel Initiation Visit Purpose Why Initiations are so critical for the Sponsor and the Site to engage in open dialogue. Sample of Initiation Agenda Detailed discussion of the protocol, including: - Overall study design and objective Study primary and secondary endpoints Inclusion/Exclusion Criteria Study Procedures Drug/Device Administration, Storage, and Handling Consent and enrollment procedures Adverse event reporting Investigator and research personnel roles/responsibilities Case report forms (Electronic or Paper) Monitoring frequency & expectations Sponsor specific forms/logs Identify potential problems and concerns Preparing for Initiation Visit Review protocol and identify potential challenges and/or concerns for discussion Become familiar with Sponsor provided documents/forms Begin maintenance of regulatory files Obtain remaining essential documents Confirm and audit supplies received Preparing for Initiation Visit Schedule tour of the site, pharmacy & lab Notify appropriate research personnel Arrange meeting room logistics Start Delegation of Authority Log & Site Signature Log and Training Log Compare Sponsor forms and Institutional Forms for similarities/differences Monitoring Visit Purpose Review progress of clinical trial and ensure protocol adherence and regulatory compliance while maintaining the rights, safety and well-being of subjects Frequency is dictated by complexity of the study and Sponsor requirements Investigator and key research personnel should be available for questions Monitoring Visit Preparation Reserve monitoring space which provides access to phone, internet, and copy/fax Schedule visits with the Investigator, Pharmacy, Laboratory and Regulatory office, if applicable Request access to medical records/source documents Complete CRFs Prepare regulatory files Allot time to meet with Monitor to discuss visit findings Preparing Source Documents Provide list of subjects with corresponding medical record number Orient monitor to medical record layout Verify all consented subjects have properly completed Informed Consent Forms and process is well documented Review drug compliance records and reconcile discrepancies Assure lab, diagnostic and radiology reports are available for review Flag sections of the chart to ease search of information Have Investigator or designee document review of laboratory results especially if out-ofrange Ensure deviations or missing information is well documented Preparing CRFs Complete CRFs up to visit date Answer queries appropriately Ensure data is accurate and verifiable by making source documents available Review Adverse Events(AE) being sure that grade, severity and attribution are assessed and documented Review concomitant medications for start/stop dates and corresponding AE entry Preparing Regulatory Files Make sure files are complete and current Retain all versions of the protocol, Investigator Brochures, laboratory certification, lab normals, FDA Form 1572, CVs, licenses, financial disclosure File IRB acknowledgement of receipt, if applicable, approval and correspondences Update signature and enrollment logs Retain Sponsor correspondences Occurs when study is complete and database is locked Final review of regulatory files Reconciliation of IP/Device Review record retention requirements Collect IRB notification of study closure Close-Out Visit Purpose Close-Out Visit Preparation Secure monitor space/room Provide regulatory files and case books Ensure Investigator and site staff are available Schedule final pharmacy visit for drug return Gather unused supplies for return/destruction Are You Ready?